Non-Small Cell Lung Cancer: Staging and Treatment
encontrar mi
What is staging for cancer?
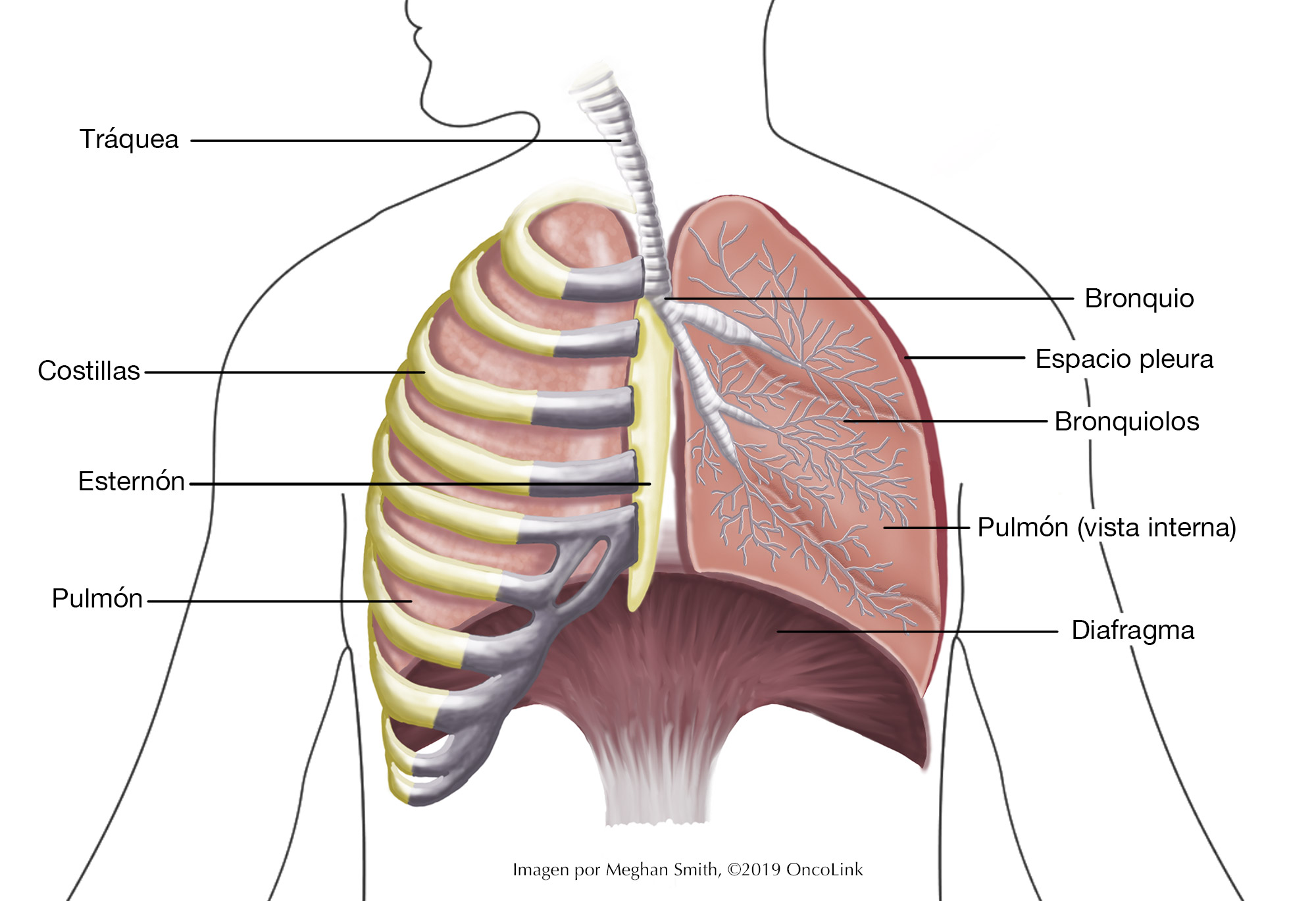
Staging is the process of learning how much cancer is in your body and where it is. For non-small cell lung cancer (NSCLC), a bronchoscopy, biopsy, chest x-ray, CT scan, MRI of the brain, and/or PET scan may be used to stage your cancer. Your providers need to know about your cancer and your health so that they can plan the best treatment for you.
Staging looks at the size of the tumor and where it is, and if it has spread to other organs.
A staging system called the “TNM system,” as described by the American Joint Committee on Cancer, helps to guide the staging of NSCLC. It has three parts:
- T-describes the size/location/extent of the "primary" tumor in the lung.
- N-describes if the cancer has spread to the lymph nodes.
- M-describes if the cancer has spread to other organs (metastases).
Your healthcare provider will use the results of the tests you had to determine your TNM result and combine these to get a stage from 0 to IV.
How is non-small cell lung cancer staged?
Staging is important because it helps to guide your treatment options.
Staging for non-small cell lung cancer is based on:
- The size of your tumor and its location in the lung(s).
- If your lymph nodes have cancer cells in them.
- If the cancer has spread to other organs (metastasis).
The staging system is very complex. Below is a summary. Talk to your provider about the stage of your cancer.
- Stage 0 (Tis, N0, M0): The cancer is only in the top layers of the airway and has not spread deeper into the lung. It has not spread to the lymph nodes or other organs.
- Stage Ia1 (T1a, N0, M0): The tumor isn’t bigger than 1 cm across in size. It hasn’t spread to the area around the lungs (pleura)and it hasn’t grown into the main parts of the bronchi. It has not spread to the lymph nodes or other organs.
- Stage IA2 (T1b, N0, M0): The tumor is between 1 and 2 cm across in size. It hasn’t spread to the area around the lungs (pleura) and it hasn’t grown into the main parts of the bronchi. It has not spread to the lymph nodes or other organs.
- Stage IA3 (T1c, N0, M0): The tumor is 2-3 cm across in size. It hasn’t spread to the area around the lungs (pleura)and it hasn’t grown into the main parts of the bronchi. It has not spread to the lymph nodes or other organs.
- Stage IB (T2a, N0, M0): The tumor has 2 of the following: (1)The tumor is 3-4 cm across in size, (2)it has grown in the main bronchus, or (3)the pleura and/or (4) is partially blocking the airways. It has not spread to the lymph nodes or other organs.
- Stage IIA (T2b, N0, M0): The tumor has 1 or more of the following: (1) it is 4-5cm across in size or (2) it has grown into the main bronchus, or (3)the pleura and/or (4) is partially blocking the airways. It has not spread to the lymph nodes or other organs.
- Stage IIB
- (T1a/T1b/T1C, T2a/T2b, N1, M0): The tumor is 3-5 cm across in size. It may or may not have spread to the main bronchus or the pleura or is blocking the airways. The cancer has spread to the lymph nodes in the lung and around the lung. These lymph nodes are located on the same side as the lung with the tumor. The cancer has not spread to other organs.
- (T3, N0, M0): The tumor is 1 or more of the following (1)it is 5-7cm across in size or (2) has grown into the chest wall, the inner pleura, the phrenic nerve, or the area around the heart (parietal pericardium), or (3) there are 2 or more separate tumors in the same part of the lung. It has not spread to the lymph nodes or other organs.
- (T1a/T1b/T1c, N2, M0): The tumor is not any bigger than 3 cm across in size and hasn’t grown into the pleural or the bronchi. It has spread to the lymph nodes near the windpipe or the space between the lungs (mediastinum). These lymph nodes are on the same side as the lung with the main tumor. The cancer has not spread to other organs.
- (T2s/T2b, N2, M0): The tumor has one or more of the following (1) it is 3-5 cm across in size, (2)it has spread into the main bronchus, or (3) it has grown into the pleura), or (4) is blocking the airways. The cancer has also spread to the lymph nodes near the windpipe or the space between the lungs (mediastinum). These lymph nodes are on the same side as the lung with the main tumor. The cancer has not spread to other organs.
- Stage IIIA
- (T1a, T1b, T1c, N2, M0): The tumor is no bigger than 3 cm across in size. It hasn’t grown into the pleura and does affect the main parts of the bronchi. The cancer has spread to the lymph nodes near the windpipe and the space between the lungs (mediastinum). These lymph nodes are on the same side as the lung with the main tumor. The cancer has not spread to other organs.
- (T2a/T2b, N2, M0): The tumor has one of more of the following: (1) It is 3-5 cm across in size, (2) it has grown into the main bronchus but isn’t within two cm of where the windpipe splits into the left/right bronchi, (3) if has grown into the pleura, 4) it is partially blocking the airways. In addition, the cancer has spread to the lymph nodes near the windpipe and the space between the lungs (mediastinum). These lymph nodes are on the same side as the lung with the main tumor. The cancer has not spread to other organs.
- (T3, N1, M0): The tumor has one or more of the following: (1) it is 5-7cm across in size, or (2) the tumor has grown into the chest wall, the inner pleura, the phrenic nerve, or the area around the heart (parietal pericardium), or (3) there are 2 or more separate tumors in the same part of the lung. The tumor has also spread to the lymph nodes in the lung and around the lung. These lymph nodes are located on the same side as the lung with the tumor. The cancer has not spread to other organs.
- (T4, N0 or N1, M0): The tumor has one or more of the following: (1) It is bigger than 7 cm across, or (2) the tumor has grown into the space between the lungs, the heart, the windpipe, the diaphragm, the esophagus, or the blood vessels near these organs, or (3) there are 2 or more tumors in different lobes of the same lung. The cancer may have also spread to the lymph nodes in the lung or near the bronchus. These lymph nodes are on the same side of the body as the tumor. The cancer has not spread to other parts of the body further from the tumor.
- Stage IIIB
- (T1a/T2a/T1c, N3, M0): The tumor is no bigger than 3 cm across in size. It has not grown into the pleura or the bronchi’s main branches. The cancer has spread to lymph nodes near the collarbone on either side of the body and to the lymph nodes near the other lung from the primary tumor site. It has not spread to other parts of the body.
- (T2a/T2b, N3, M0): The tumor has one or more of the following: The tumor has one or more of the following (1) it is 3-5 cm across in size, (2)it has spread into the main bronchus, (3) it has grown into the pleura), or (4) is blocking the airways. The cancer has spread to lymph nodes near the collarbone on either side of the body and to the lymph nodes near the other lung on the other side of the body from the primary tumor site. It has not spread to other parts of the body.
- (T3, N2, M0): The tumor has one or more of the following: (1) it is 5-7cm across in size, or (2) the tumor has grown into the chest wall, the inner pleura, the phrenic nerve, or the area around the heart (parietal pericardium), or (3) there are 2 or more separate tumors in the same part of the lung. The cancer has also spread to the lymph nodes near the windpipe and the space between the lungs (mediastinum). These lymph nodes are on the same side as the lung with the main tumor. The cancer has not spread to other organs.
- (T4, N2,M0): The tumor has one or more of the following: (1) It is bigger than 7 cm across, or (2) the tumor has grown into the space between the lungs, the heart, the windpipe, the diaphragm, the esophagus, or the blood vessels near these organs, or (3) there are 2 or more tumors in different lobes of the same lung. The cancer has also spread to the lymph nodes near the windpipe and the space between the lungs (mediastinum). These lymph nodes are on the same side as the lung with the main tumor. The cancer has not spread to other organs.
- Stage IIIC
- (T3, N3, M0): The tumor has one or more of the following: (1) it is 5-7cm across in size, or (2) the tumor has grown into the chest wall, the inner pleura, the phrenic nerve or the area around the heart (parietal pericardium), or (3) there are 2 or more separate tumors in the same part of the lung. The cancer has spread to lymph nodes near the collarbone on either side of the body and to the lymph nodes near the other lung on the other side of the body from the primary tumor site. It has not spread to other parts of the body.
- (T4, N3, M0): The tumor has one or more of the following: (1) It is bigger than 7 cm across, or (2) the tumor has grown into the space between the lungs, the heart, the windpipe, the diaphragm, the esophagus or the blood vessels near these organs, or (3) there are 2 or more tumors in different lobes of the same lung. The cancer has spread to lymph nodes near the collarbone on either side of the body and to the lymph nodes near the other lung on the other side of the body from the primary tumor site. It has not spread to other parts of the body.
- Stage IVA
- (Any T, Any N, M1a): The tumor can be any size and may have grown into the pleura, bronchi, mediastinum, or other nearby parts of the body. It may have spread to the lymph nodes. It may also (1) have spread to the other lung or (2) be in the fluid around the lung (pleural effusion) or (3) in the fluid around the heart (pericardial effusion).
- (Any T, Any M, M1b): The tumor can be any size and may have grown into the pleura, bronchi, mediastinum, or other nearby parts of the body. It may have spread to the lymph nodes. The tumor has also grown to one space away from the chest. This could be a lymph node in another part of the body, the brain, the liver, or the bones.
- Stage IVB
- (Any T, Any M, M1c): The tumor can be any size and may have grown into the pleura, bronchi, mediastinum, or other nearby parts of the body. It may have spread to the lymph nodes. The tumor has also grown to more than one space away from the chest. This could be a lymph node in another part of the body, and/or the brain, the liver, or the bones.
How is non-small-cell lung cancer treated?
Treatment for NSCLC depends on the stage of your cancer. If you smoke, quit as soon as possible. Smoking may lessen how well your cancer treatments work and can make the side effects of treatment worse.
Your treatment may include some or all of the following:
- Surgery.
- Chemotherapy.
- Targeted therapy.
- Immunotherapy.
- Radiation therapy.
- Palliative treatment.
- Clinical trials.
Surgery
Surgery can be used in the treatment of NSCLC. The goal of surgery is to remove as much of the cancer as possible. There are many different surgical procedures used in the treatment of NSCLC. These procedures include:
- Wedge resection: the surgeon will remove a small part of the lung affected by the cancer.
- Lobectomy: the surgeon will remove the lobe of the lung affected by the cancer.
- Pneumonectomy: the surgeon will remove the entire lung.
The type of surgery you may have depends on the location and size of your tumor and your overall health. You may also have pulmonary function tests (PFTs) before surgery to make sure you can tolerate the surgery.
Surgery may also be used if the cancer has spread (metastasis). This might include the removal of tumors that have spread to parts of the body like the brain, spine, or adrenal gland.
Chemotherapy
Chemotherapy is the use of anti-cancer medicines that go through your whole body. These medicines may be given through a vein (IV, intravenously) or by mouth. Chemotherapy for NSCLC may be used with immunotherapy and/or radiation therapy. What treatment you receive and how often you have treatment will depend on the stage of your cancer.
The medications used to treat NSCLC include: cisplatin, carboplatin, pemetrexed, paclitaxel, docetaxel, etoposide, gemcitabine, and vinorelbine. Your provider will work with you to determine the best chemotherapy plan for your cancer and the potential side effects of your treatment.
Targeted Therapy
Some cancers have biomarkers that help providers to focus your treatment on certain genetic mutations or receptors in your tumor. These treatments are called targeted therapies. They target those genetic mutations. Your provider will test your tumor for these markers.
Targeted therapies used in the treatment of specific genetic mutations include:
- EGFR positive: Afatinib, erlotinib, dacomitinib, gefitinib, ramucirumab, bevacizumab and osimertinib.
- ALK positive: Alectinib, brigatinib, ceritinib, crizotinib and lorlatinib.
- KRAS G12C mutation: Sotorasib, adagrasib.
- ROS1 positive: Ceritinib, entrectinib, lorlatinib, and crizotinib.
- BRAF V600E positive: Dabratinib and trametinib, vemurafenib.
- RET Rearrangement positive: Selpercatinib, pralsetinib, cabozantinib.
- MET Exon 14 Skipping mutation: Crizotinib, capmatinib, tepotinib.
- ERBB (HER2) mutations: Ado-tratuzumab emtansine, fam-trantuzumab deructecan-nxki.
- NTRK Gene Fusion positive: Larotrectinib, entrectinib.
- Anti-angiogenesis agents: Bevacizumab and ramucirumab
- PD-L1: cemiplimab-rwlc, tremelimumab-acti, ipilimumab.
Your provider will talk to you about if targeted therapy can be used to treat your cancer and any side effects you may have.
Immunotherapy
Immunotherapy medications work with the immune system to kill cancer cells. Immunotherapy medications that may be used in the treatment of NSCLC are nivolumab, ipilimumab, pembrolizumab, atezolizumab, and durvalumab. Your provider will talk to you about if these medications will help treat your cancer and what side effects you may have.
Radiation
Radiation is the use of high-energy X-rays to kill cancer cells. For NSCLC, radiation may be used before surgery to shrink the tumor and make removing it easier. It may also be used with chemotherapy/targeted therapy/immunotherapy at the same time. Radiation to the chest may also be used to help prevent the cancer from coming back.
Sometimes, lung cancer spreads to other parts of the body (metastasis). Radiation may be used to treat the spread of lung cancer to these places as well as to help reduce pain from the cancer spreading.
Palliative Treatment
Palliative therapy is used to relieve symptoms that are caused by the cancer. It does not cure the cancer. There are many options for palliative treatments including chemotherapy, radiation, surgery, stent placement, laser therapies, and removal of extra fluid from around the heart or lungs. Talk to your provider about your options for managing your symptoms.
Clinical Trials
You may be offered a clinical trial as part of your treatment plan. To find out more about current clinical trials, visit the OncoLink Clinical Trials Matching Service.
Making Treatment Decisions
Your care team will make sure you are included in choosing your treatment plan. This can be overwhelming as you may be given a few options to choose from. Take the time to meet with different providers and think about your options and what is best for you. This is a personal decision. Friends and family can help you talk through the options and the pros and cons of each, but they cannot make the decision for you. You need to be comfortable with your decision – this will help you move on to the next steps. If you ever have any questions or concerns, be sure to call your team.