Module 14: Pediatric Proton Therapy
This content is no longer being maintained or updated. While it is available for review, please keep in mind the dates of creation, since some information may no longer be current.
For up-to-date proton therapy education designed for healthcare providers, please visit the Penn Radiation Medicine Institute. Current training and educational materials are available through our partnered and purchased programs.
encontrar mi
The treatment of childhood tumors is an area where proton therapy may significantly reduce the acute and long-term complications associated with conventional radiation therapy. Pediatric cancer treatment has significantly improved over the past few decades and now 5 year overall survival is approximately 80%. There are approximately 270,000 survivors of childhood cancers in the United States alone. However, the pediatric population is exquisitely sensitive to the effects of radiation therapy. In this group of patients, cure is not enough. Long-term sequelae including growth abnormalities, second malignancies, neurologic complications, reduced IQ, cardiac and pulmonary toxicities, and infertility may all be reduced with the use of proton therapy. Up to 2/3 of patients will develop long term toxicity, some of which will be fatal. These toxicities can cause a great deal of emotional and financial stress for the patient. It is known that age at the time of radiation as well as the dose to which the child was treated are important risk factors. Specifically, developing tissues are more sensitive to radiation, hence there can be detrimental effects even at low doses. With improved outcomes there is greater concern regarding treatment induced cancers as there are a greater number of potential years of life during which the secondary malignancy can manifest. Technical improvements have focused on decreasing toxicity while maintaining good outcomes. Numerous clinical trials have attempted to eliminate radiation; however radiation still plays an important role in more than half of pediatric patients. While IMRT can deliver dose with greater conformally, it does bathe a significant amount of normal tissues with low doses of radiation, which can be significant in younger patients.
Proton therapy can reduce the integral radiation dose by 2-3 fold compared with any type of photon based therapy. It is proposed that, due to the decreased normal tissue dose, proton treatment will lead to the development of less secondary malignancies. Several studies have shown that the rate of secondary malignancies in pediatric patients treated with conventional radiation is currently about 3-13% of patients. (Click here to learn more about secondary malignancy)
The largest clinical experiences with proton therapy in pediatric patients in the United States comes from Massachusettes General Hospital (MGH) and Loma Linda University. At MGH, they have approximately 60 patients on proton treatment per day on two gantries, of which 17% are pediatric patients. The following sections discuss the data available regarding proton therapy for a variety of pediatric tumors, broken up by site, with the majority of the data coming from the two above mentioned institutions.
Sarcomas
Orbital Rhabdomyosarcoma (RMS)
Rhabdomyosarcomas are tumors of connective tissue that arise from skeletal tissue, and are the most common pediatric tumor. Rhabdomyosarcomas that occur in the orbit have a very good prognosis, and radiation is an effective treatment that can avoid removal of the eye (enucleation) and preserve vision. However, the proximity of the orbit to the lacrimal gland and lens can lead to late side effects. Two patients with orbital rhabdomyosarcoma were treated with protons at Loma Linda in 1995 and 1996 to a dose of 50 and 55 CGE. At 3.4 and 2.5 years after protons, both children were alive and without recurrence. Both had with excellent visual acuity without cataracts, but one had mild enophthalmos.
Seven pediatric patients have undergone proton irradiation for orbital RMS at MGH, with six years of follow-up. All seven are currently alive without evidence of disease. One patient did experience local failure, but was salvaged with enucleation and stereotactic radiosurgery. All other patients have excellent vision. It should be noted that 50% of patients experience visual impairment in those cohorts treated with conventional photons. Patients treated at MGH have been noted to have mild orbital asymmetry, but have experienced no development of cataracts, keratosis, neuroendocrine issues, or painful dry eye at this point. Dosimetrically, the greatest benefit of proton treatment over photon treatment has been in sparing of the brain and contralateral orbital structures.
Parameningeal Rhabdomyosarcoma
"Parameningeal" rhabdomyosarcoma arise in certain sites in the head, including the infratemporal fossa, pterygopalatine fossa, middle ear, mastoid, nasal cavity, nasopharynx, and paranasal sinuses. Unlike rhabdomyosarcomas that originate in the orbit, parameningeal rhabdomyosarcoma has a poor prognosis. The location of these tumors makes surgical resection difficult, and standard treatment includes chemotherapy and radiation.
Several investigations regarding proton treatment of parameningeal RMS have been carried out at MGH. A dosimetric study of 10 patients comparing intensity modulated photon radiotherapy versus proton radiotherapy demonstrated increased dose conformality with associated sparing of normal tissues such as the whole brain, brainstem, pituitary gland, and hypothalamus in favor of protons. Only the ipsilateral parotid gland and cochlea did not receive decreased dose with proton radiation. A retrospective study of late effects for patients treated for parameningeal RMS at MGH was presented at the Particle Therapy Cooperative Group Meeting. In this study, 17 patients who had been treated for parameningeal RMS between 1996 and 2005 were evaluated. Median age at time of treatment had been 3.4 years (range 1.5 – 17.6 years), and RMS histology was embryonal in 11 patients, alveolar in four, and undifferentiated in two. Ten patients had intracranial extension. Median treatment dose was 50.4 Cobalt Gray Equivalents (CGE) (range 50.4 – 55.8 CGE), and median follow-up was 4.3 years. Three year overall survival in this group was 61%. Late effects were compared to those published by other groups using photon based irradiation to treat parameningeal RMS, namely the IRS II/ III, which used a combination of three-dimensional conformal radiotherapy (3DCRT) and intensity modulated radiotherapy (IMRT) to treat 213 patients (Raney, 1999), results from Memorial Sloan Kettering Cancer Center using IMRT to treat 22 patients (Wolden, 2005), and a series from Iowa University again using a combination of 3DCRT and IMRT to treat 17 patients (Paulino 2000). When late effects were compared, 20% of patients treated with protons developed decreased height as opposed to 48 – 60% of those treated with photons. Facial hypoplasia developed in 60% of patients treated with protons versus 73 – 97% of those treated with photons. Dental issues were present in 30% of patients treated with protons and 100% of those treated with photons. Cognitive deficiencies developed in 10% of those treated with protons versus 20 – 50% of those treated with photons. No visual changes, hearing loss, or second malignant neoplasms were described in patients treated with protons, whereas these effects developed in 9 - 82%, 17 -75%, and 2 – 9%, respectively, of those treated with protons. The benefits of proton based treatment for parameningeal RMS seem clear, if not in terms of disease outcome than certainly with respect to late effects. One important consideration, is that as the tumor shrinks, it can alter the proton path length considerably. Furthermore, the tumor-tissue-air interfaces may also change during the course of treatment. Hence, adaptive radiotherapy is particularly important in treatment of these tumors with protons.
Central Nervous System Tumors
Malignant tumors that arise in the base of the skull, typically chordomas, chondrosarcomas, and rhabdomyosarcomas, are uniquely challenging due to their location and proximity to critical structures. Surgical resection is typically incomplete. Despite treatment with post-operative photon radiation, these tumors will come back 60-75% of the time. One possible reason for this poor outcome is that the with standard photon radiotherapy, the total dose is limited by the dose to sensitive normal tissues, such as the optic nerves, the optic chiasm, and the pituitary gland. At the Harvard Cylclotron, 67 adults with chordomas or low-grade chondrosarcomas of the base of skull or cervical spine were treated with proton radiotherapy resulting in very good local control (89% at 3 years). This work has established the role of protons in base of skull tumors in adults, but data in children are more limited.
Between 1981 and 1990, 18 children with base of skull or cervical spine chordomas were treated at the Harvard Cyclotron. After surgery, the children were treated with a mix of photons and protons to a median dose of 69 CGE. After a median follow-up of 72 months, the 5-year actuarial survival was 68% and the disease free survival was 63%. Of the 18 children, four had radiation-related morbidity (growth hormone deficits, temporal lobe necrosis, and temporalis muscle fibrosis). Additional children with chordoma were treated with protons in Boston (73 patients total), and with a mean survival of over 7 years, overall survival was 81%. Despite the observation that chordoma in children has a better prognosis than in adults, children with poorly differentiated tumors had very poor outcomes compared to those with well differentiated tumors. The Boston experience was updated in 2006 (PTCOG45, Houston, TX) and showed that children with chordoma treated with protons experienced a local control rate of 80% at both 5 and 10 years without gender differences.
At Loma Linda, 20 children with chordomas, chondrosarcomas, and rhabdomyosarcomas of the base of skull were treated with protons between 1992 and 1999. They were treated with doses between 50.4 and 78.6 CGE. The results with protons compared favorably with photon treatment, with 5-year local control of 72% and overall survival of 56%. Longer follow-up will be needed to determine the benefit on bone growth & cosmetic outcome.
Links to reviews of recent abstracts and presentations regarding proton therapy for base of skull tumors:
- Proton radiation therapy in the management of prediatric base of skull tumors
- Proton Radiotherapy for Pediatric Ewing's Sarcomas: Initial Clinical Outcomes of 29 Patient
Craniopharyngioma
Craniopharyngiomas are benign tumors consisting of calcium deposits mixed with cysts arising from Rathke's pouch, which is near the pituitary gland. Resection is frequently incomplete, and the addition of radiotherapy can decrease the chance of the tumor coming back. Incomplete resection plus radiation together can prevent the tumor from recurring in 70-90% of patients. Photon radiotherapy is effective, but necessarily requires irradiation of critical normal tissues. The most common late complication following treatment for craniopharyngioma is hypopituitarism. Other late complications include vision and neurocognitive problems. It can be difficult to tell what specifically caused the late effects in this disease which can be due to surgery, radiation, or damage from the tumor itself.
At Loma Linda, 16 patients with craniopharyngioma (age 7-34 yr) were treated with protons after at least one resection. They were treated with 50.4-59.4 CGE (1.8/day), and 14/15 patients had not recurred after 25 months of follow-up. There were few acute side effects from treatment, but there were late effects observed in four patients, namely panhypopituitarism, one stroke, and one meningioma that occurred out of the proton-field in the patient that had previous photon radiation.
A cohort of 24 pediatric patients have been treated with proton radiotherapy at MGH from 2001 – 2007. Median dose was 52.2 CGE (range 52.2 – 54 CGE), and treatment was delivered using a four-field approach. Although 24% of patients required replanning during the course of radiotherapy for cystic tumor enlargement, outcomes have been excellent: Median follow-up is 50 months, and local control rates are 100%.
There have also been studies in Europe which suggest that dose escalation is possible in craniopharyngioma while reducing the dose to surrounding normal tissues.
Links to reviews of recent abstracts and presentations regarding proton therapy for craniopharyngiomas:
- Podcast from Opportunities in Proton Therapy: Craniopharyngioma
- Craniopharyngioma: Early Response Evaluation of Patients Treated with Protons at the Midwest Proton Radiotherapy Institute in Bloomington, Indiana
- Dose Escalation in Pediatric Brain Tumors with Radiation Therapy. A dosimetric Evaluation on The Potential Role of Proton Beam Therapy
- Craniopharyngioma in Childhood: Proton Radiotherapy and Treatment Planning With Special Attention to the Cyst Size
Gliomas
Astrocytomas are the most common primary brain tumor in children. They arise from glial cells, which are cells in the brain that support the neurons. Depending on the grade and location of the tumor, children can do very well (over 95% survival at 5 years after complete resection of low-grade tumors) or very poorly (5-year survival of 15-30% for high grade tumors and less than 10% for pontine tumors). Depending on the location, grade, and age of the patient, tumors are treated with a combination of surgery, radiation, and/or chemotherapy. The long-term side effects of radiation are related to the dose and the volume that is treated, which can be extensive in astrocytomas. Therefore, long-term neurocognitive complications, such as deficits in memory, learning, and social/emotional adjustment may by minimized by limiting dose to normal brain tissue. To explore this concept, dosimetry studies comparing photon with proton treatment for astrocytomas have shown less normal brain would be treated with proton therapy, even if the tumor dose were increased.
At Loma Linda, 27 children with progressive, recurrent, or residual low-grade astrocytomas were treated with protons to a dose of 50.4-63 CGE. With a mean follow-up of 3.3 yrs, the local failure rate was 6/27 (22%). The treatment was well tolerated, and all children with local control maintained their performance status. All of the patients with optic pathway tumors (6) who started with useful vision maintained or improved their visual status.
30 pediatric patients have been treated with proton radiotherapy at MGH from 1995 – 2006. Median age was 10 years (range 2 – 21 years); 60% of tumors were WHO grade 1, 20% WHO grade 2, and 20% unbiopsied. Median tumor dose was 52.2 CGE, and median follow-up 44 months. At this point, two patients have developed local recurrences (one anaplastic astrocytoma and one pleomorphic xanthoastrocytoma) -- disease free survival is 93%, and overall survival 100%. With respect to late effects, two patients, both with initial disease presentation at less than two years of age and with tumor compression of the circle of Willis, have developed Moya Moya syndrome. Both are alive and well, but have required surgical intervention. Six patients have undergone baseline and follow-up neuropsychiatric evaluation spanning approximately two years. Over that time period, no significant IQ loss has been noted. Of all 30 patients, 23% were noted to have neuroendrocrine deficits at the time of presentation; an additional 23% have developed new neuroendocrine deficits since completing treatment.
Optic pathway gliomas account for 1-5% of all childhood gliomas, and their location presents a particular neurosurgical challenge. Resection of anterior tumors can cause blindness in the affected eye, and resection is not always feasible for tumors extending posteriorly. At Loma Linda, seven children with optic pathway gliomas were treated with protons to a dose of 54 GCE 12. With 3.1 years of follow-up, all the patients were alive and free of recurrence. Visual acuity was preserved in all the patients that presented with useful vision.
Neuroblastoma
The most common extra-cranial solid tumor in childhood is neuroblastoma. It is a tumor of nerve tissue that most commonly arises in the adrenal glands in the abdomen, but can start in other locations. At Loma Linda, a 4 year-old child with neuroblastoma of the right adrenal gland underwent protons to a dose of 25.2 CGE. A boost region was treated to 34.2 CGE. The calculated dose to surrounding normal tissues in the abdomen was very low. The child tolerated treatment without any acute intestinal, hepatic, or renal toxicity, and experienced only mild redness of the posterior para-spinal skin.
Medulloblastoma
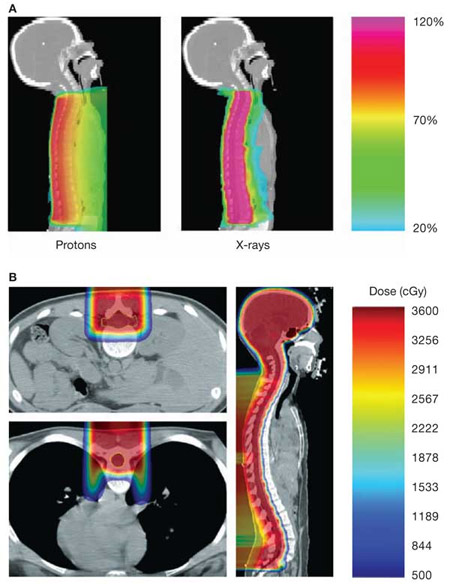
Medulloblastomas are primitive neuroectodermal tumors that arise in the cerebellum. They are treated with resection, and post-operative radiation is used to decrease the risk of recurrence. Medulloblastomas have a tendency to seed the craniospinal fluid, and therefore, comprehensive craniospinal irradiation is employed. Unfortunately, using conventional photon radiotherapy, a large volume of normal tissue is treated as well, including the heart, lung, bowel, gonads, and vertebral bodies. Proton radiotherapy offers a major potential advantage over conventional photons as the lack of exit dose can prevent treatment of tissue deep to the spine (Figure 1. Yock TI et al. Nature Clinical Practice Oncology (2004) 1, 97-103) (Figure 2. Krejcarek SC et al. Int J Radiat Oncol Biol Phys. 2007 Jul 1;68(3):646-9.). Proton therapy also can prevent radiation dose to normal tissues for the cranial boost, especially for sensitive structures such as middle ear.
Figure 1. Example of medulloblastoma treated with proton therapy
Figure 2. Example of a 14 yo treated with a primitive neuroectodermal tumor treated to a craniospinal field using proton therapy. Note that the fatty changes in the vertebral bodies correlate with the distal edge of the proton beam
At Loma Linda, three children with medulloblastoma were treated with craniospinal proton irradiation to a dose of 36 CGE with a18 CGE posterior fossa boost. No clinically significant blood count depression was seen, and only Grade 2 dermatitis was observed. One child was followed for 3 years and did not show any evidence of scoliosis. More patients and longer follow-up will be needed to fully realize the relative benefit PRT in medulloblastoma.
There appears to be a benefit of proton irradiation in delivering both phases of medulloblastoma radiotherapy – the craniospinal portion and the posterior fossa boost. The complete lack of exit dose associated with craniospinal proton irradiation, allows the avoidance of dose to the heart, lung, bowel, and ovaries. There is also the potential to deliver dose only to the thecal sac, rather than the entire vertebral body, in children whose growth is complete. With regard to the second phase of medulloblastoma treatment, the posterior fossa boost, proton irradiation allows more normal brain tissue to be spared, including the temporal lobes and cochlea. Protection of these structures is particularly important since patients usually receive platinum-based chemotherapy. Additionally, use of protons may allow significant reduction in exit dose, sparing large portions of the supratentorial brain. This is true both when children receive boost dose to the entire posterior fossa, and when the boost is delivered only to the tumor bed. Although techniques vary somewhat between institutions, the issue of medulloblastoma boost is currently under investigation in a phase III Children's Oncology Group study. With regards to partial brain treatment, MGH employs a method of delivery using posterior oblique fields rather than the traditional opposed lateral beams. This adjustment has been made to allow more complete sparing of the lenses while still allowing coverage of the cribiform plate.
Links to reviews of recent abstracts and presentations regarding proton therapy for medulloblastomas:
- Advantage of protons compared to IMRT in the treatment of medulloblastoma
- Podcast from Opportunities in Proton Therapy: Medulloblastoma
Retinoblastoma
Retinoblastoma is the most common malignant eye tumor of childhood, although still very rare. Because it is associated with a mutation in the tumor suppressor gene, Rb, 25% of cases are bilateral. Cure rates are generally high, so minimization of adverse effects, especially vision loss is an important goal. Although enucleation can be an effective treatment, vision-preserving treatments are desired. Chemotherapy with vincristine and carboplatin is often supplemented with radiation therapy.
In Belgium, at the cyclotron of Louvain-la-Neuve, three patients with retinoblastoma were treated with protons. The short-term outcomes were comparable to treatment with photons, but long-term follow-up with more patients would be needed to determine if the improved dosimetry decreases late toxicity.
Secondary Malignancies
The relative risk of secondary malignancies is higher in children. Children have an approximately ten fold higher sensitivity to radiation. Prior studies have shown that exposures on 0.5 to 1 Sv leads to a 7-15% increase in the risk of developing secondary cancers in children treated with radiation. Children are smaller with organs that are more compact which may increase organ exposure to radiation. Children are more likely to have genetic defects which may make them more susceptible to developing secondary malignancies.
Dr. Eric Hall recently published a paper which estimated dose outside of the fields edge for both IMRT and protons, which ahs been quite controversial (Int. J. Radiation Oncology Biol. Phys., Vol. 65, No. 1, pp. 1–7, 2006). Dr. Hall recently addressed this at Radiation Research Meeting in San Francisco in 2007. He noted that dose outside the field's edge approximately doubles in patients who receive IMRT compared with 3D conformal therapy. He also noted that the dose outside the field's edge from a scanning proton beam is less than with IMRT. This is important as many children are treated with IMRT to achieve greater conformality, Dr. Hall's findings suggest that scanning beam protons may not only improve conformality but also reduce whole body dose from secondary radiation.
Dr. Hall also reported data on a proton beam utilizing scatter foils obtained from Harvard which he noted was old. Specifically, the neutron dose estimates were based on data collected from facilities where the scatter foils were located much closer to the patient than at newer facilities. At present the foils are located much further from the patient and contribute much less neutron dose. Secondly, the data the data used by Dr. Hall was based on the "worst case scenario" where a large field is used to treat a small target with the majority of the field blocked with an aperture, resulting in a large neutron scatter dose. The true value for the dose outside of the field's edge for proton beams utilizing a scatter foil may be 9 fold less than reported in Dr. Hall's paper which is close to the doses seen in IMRT treatment. However, Dr. Hall believes that the relative biological effect (RBE) of neutrons used in the estimate of dose was too low at 10 and believes that an RBE of 30 would be more appropriate. This puts the dose outside the fields edge of proton beams which use scatter foils above that of IMRT, however it is still less than the dose Dr. Hall initially published. He reiterated that the bulk of neutrons created in proton therapy are from the scatter foils and hence the use of a scanning beam, which does not require the use of scatter foils, should significantly reduce neutron production. Taken together, it appears that the preferred proton treatment modality for pediatric patients in with a scanning proton beam. Of note there has never been a difference shown between the RBE of protons in children versus the RBE seen in adults.
Dr. Hall has also published a recent estimate of the risk of secondary malignancy related to neutron exposure from passive scatter proton therapy in the pediatric population. He used an RBE of 25 for the neutrons in his calculations to estimate exposure dose. This dose was extrapolated to A-bomb survivors who received an equivalent dose and their subsequent lifetime risk for developing a cancer. They found that the overall lifetime risk for cancer for a 15 year old boy was approximately 5% and for a 15 year old girl it was approximately 10%. It would be lower for older patients and higher for younger patients. Dr. Hall concluded that these rates were not significantly different compared with IMRT, however, he also points out that a scanning beam system would greatly reduce neutron exposure. Hence, since most of these children would be treated with IMRT, it appears that passive scatter protons do not produce an appreciable increase in secondary malignancy, however, it appears that scanning beam would be a significant improvement over both passive scatter protons and IMRT.
A recent retrospective study from MGH supports Dr. Hall's findings. The study was a retrospective matched study that compared patients treated at the Harvard cyclotron facility from 1974-2001 with patients treated with photon therapy extracted from the SEER database. At total of 503 patients treated with protons were matched to one to three patients treated with radiation from the SEER database. Patients treated with a small proton field were not included in this study. Patients from age 1-90 were included in this study with the median age being 62. Median follow up for proton patients was 6.8 years versus 5.2 years for patients treated with photons. All patients were matched for age, year of treatment, histology and site of treatment. The primary outcome of this study was secondary malignancy. It is important to note that the majority of patients treated with protons also received photons for part of their treatment course (typically 20% of the treatment). The results from the study demonstrated that 32 patients (6.4%) treated with protons developed secondary malignancies as compared to 66 patients (13.1%), who received photons and that this difference was statistically significant. Limitations of this study include that the study was retrospective and used SEER data (which has its own limitations) as well as the fact that the majority of patients treated with protons received about 20% of their treatment with photons. However, this study does present the first comparative analysis of secondary cancer incidence rates between protons and photons and it appears that patients treated with protons did have a significantly lower rate of secondary malignancy compared with matched patients treated with photons.
Quality of life, sociological impact
Well-recognized side effects of conventional photon irradiation of the brains of young children include neuropsychologic and intellectual deficits. The side effects vary directly with the volume of brain tissue irradiated and the dose of radiation delivered. By decreasing both the volume and dose of radiation to normal brain tissue through the use of protons, these side effects should be reduced.
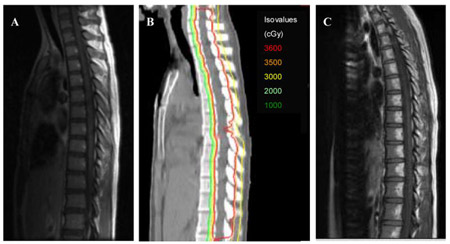
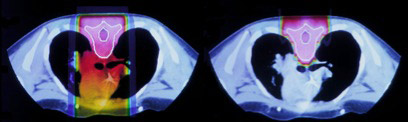
Figure 3. (Miralbell R, et al. IJROBP 2002;54:824-9) shows the difference in dose distribution between X-rays and protons for the treatment of the spinal axis is children with medulloblastoma. Figure 4 (Saran F. European Journal of Cancer 2004;40(14):2091-105) shows the difference in dose to the cochlea and temporal lobes for a posterior fossa boost in a child with medulloblastoma.
Figure 3. Example of dose to the heart for a photon spinal field (left) versus a proton spinal field (right)
Figure 4. Example of reduced cochlear and temporal lobe dose in a posterior fossa boost for medulloblastoma using protons (left) and IMRT (right)
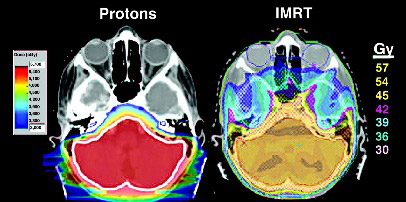
Neurocognitive delays are certainly at the forefront of consideration of radiotherapy technique in treatment of CNS tumors in children. Researchers from St. Jude's Children's Research Hospital have performed IQ modeling studies demonstrating that age and brain radiation dose are significant factors in determining IQ deficits secondary to radiotherapy. Further studies from St. Jude's Children's Hospital suggest that for patients with MB, OPG, and CR, proton therapy can reduce dose to normal parts of the brain to the point where these is a clinically significant difference in IQ change favoring proton therapy (based on models of doses effect on cognitive function). This study also examined the potential dose to the hypothalamic pituitary axis and found that in EP and MB, proton therapy may reduce dose, potentially decreasing the risk for growth hormones deficiency, hypothyroidism, gondaotropin insufficiency and adrenal insufiiciency. This study also demonstrated that the cochlear could be sparred to a greater degree with protons as compared to photon based treatment plans.
Links to reviews of recent abstracts and presentations regarding proton therapy for pediatric cancers:
- Overview: Proton Radiotherapy in Pediatric Tumors
- Particle and Proton Radiotherapy in Treatment of Pediatric Oncology Patients
Anesthetization and Immobilization
Given the need to limit the dose as much as possible to normal tissues, patient immobilization is crucial in the pediatric populations. As discussed previously, protons require even more stringent immobilization as the tighter margins increase the risk of marginal misses due to patient positioning errors. Systematic errors in setup likely lead to the greatest risk of a marginal miss and hence an immobilization device which is both precise and highly reproducible is required. This is further complicated in the pediatric patient because of the greater difficulty they have in limiting their motion during treatment. Unfortunately, this can be compounded because generally firmer, more elaborate immobilization devices are more effective, but are also often more difficult for a pediatric patient to tolerate, particularly for those devices used in head and neck and CNS tumors.
Pretreatment teaching and psychoeducational intervention have been shown to help with the tolerability of immobilization devices, however, often times anesthesia is required to adequately sedate the pediatric patient. A recent study from St. Jude's Hospital demonstrated that 62% of pediatric patients from 2-7 years of age required some sort of sedation for CT simulation. 92% of those requiring sedation required propofol, 6% received moderate IV sedation and 2% had oral sedatives. Younger age and higher anxiety and distress at baseline as well as prior to treatment were associated with an increased risk of anesthesia. Anesthesia presents several challenges. The patient often needs to be prone and the cardiac and respiratory equipment must be monitored from outside of the room. Furthermore, due to the size of the proton gantry, treatment table, and onboard imaging equipment it can be difficult to position anesthesia equipment in such a way that it does not interfere with treatment without specialized proton treatment rooms. As the patient will be undergoing daily anesthesia, it is important that the anesthetic maintain spontaneous ventilation as much as possible to decrease airway trauma, is easily titratable, and has a rapid recovery. Generally, given these needs, propofol is the anesthetic of choice at many institutions. A recent study performed at St. Jude's Hospital demonstrated a 1.3% complication rate (defined as oxygen desaturation, apnea, airway obstruction, minor airway complication and hemodynamic compromise) related to anesthesia for radiation. Risk factors for complications included procedure duration, total propofol dose, anesthesia with multiple agents, and simulation (as compared to radiation treatments).
Neuro-Psychiatric and Cognitive Testing in Follow-up
Cranial radiation has the potential to affect attention span, memory, executive function and the rapidity with which information is processed. Diminished IQ can be seen, particularly with problems related to math and reading. Behavioral changes may occur as well. There are several factors which affect the severity of the deficits. High doses can be related to worse outcomes, with a greater decline in IQ. Age at the time of treatment also affects the severity, with younger patients doing worse than older ones. New symptoms can develop as the time from radiation treatment increases. Additionally, radiation used in conjunction with chemotherapy can also cause worse neurocognitive deficits. After cranial radiation, patients should be evaluated yearly to track their educational progress and intellectual development. A formal neuropsychological evaluation should be performed at the start of follow up to obtain a baseline. In the event that there are clinical signs of intellectual or educational impairment, neuropsychological evaluation should be repeated. Neuropsychological evaluation should include testing of the rate of information processing, visual and motors integration, attention, memory, comprehension of verbal instructions, verbal competency, and evaluation of executive functions. In the event that neuropsychological deficits are found, the patient should be referred for additional social and education skill training, if applicable at their school. Additionally, some patients may benefit from treatment with stimulants, though caution should be used when prescribing these as patients may be more sensitive to these mediations after cranial radiation. Neuropsychiatric rehabilitation may also be of benefit.
Endocrine Testing
A number of endocrine abnormalities can occur due to cranial radiation particularly when the targets are the pituitary and hypothalamus. Risk factors associated with increased rate of endocrine disorders includes higher radiation doses, younger age at the time of treatment, prior surgery to the suprasellar region, and treatment with greater than 18 Gy. Retardation of growth can occur and should be monitored for every 6 months after cranial radiation until the completion of growth at which time it can be monitored annually. This includes recording height, weight and BMI of the patients. Additionally Tanner staging should be evaluated every 6 months until the patient is sexually mature. Thyroid function tests should also be obtained. In the event the child is felt to be growing poorly, x-rays can be obtained to determine the bone age of the child. An endocrinology consult is recommended in children who fall below the third percentile on a growth chart or who drop more than 2 percentile rankings. Additionally, if there is no pubertal growth spurt or if the patient grows less than 4 to 5 cm in a year during childhood endocrinology should be consulted. Growth hormone supplementation should be considered in patients who are found to be deficient.
Precocious puberty may also occur after cranial radiation and is more likely with doses of radiation greater than 18 Gy. The physical evaluation is similar to those above for monitoring of growth. Additionally, FSH, LH, testosterone and estradiol levels can be checked if there is a clinical suspicion of accelerated puberty or growth.
Radiation doses greater than 40 Gy delivered to the cranium can also cause hypoprolactinemia. Patients who have had surgery to the hypothalamic area or higher doses of radiation (greater than 50 Gy) are at increased risk. Symptoms include galactorrhea, menstral irregularities or decreased libido. These symptoms should be assessed yearly. In patients with any of the above symptoms, a prolactin level should be drawn. In the event increase prolactin levels are found a CT of the brain looking for a pituitary adenomona is recommended
Radiation to the cranium can also result in hypothyroidism. The risk for hypothyroidism increases with dose of radiation. The classic symptoms for hypothyroidism may be seen including fatigue, weight gain, dry skin, cold intolerance, depression and brittle hair. These symptoms should be checked for on a yearly basis. A TSH and a free T4 level should be checked at least yearly and more often during periods of rapid growth. Women who are interested in pregnancy should have thyroid levels checked prior to attempting pregnancy. If clinical suspicion is high TSH surge testing should be considered.
Gonadotropin deficiency can also occur, particularly with higher doses of radiation to the cranium. Symptoms include sexual dysfunction or pubertal loss and these should be assessed yearly. In younger patients the Tanner stage and the testicular volume should be checked yearly. FSH, LH, and testosterone levels should be drawn as a baseline at 14 years of age and redrawn as clinically indicated. In the event of infertility a semen analysis may be ordered. In patients found to be hypogondal, hormone replacement should be considered. Referral to a reproductive endocrinology should be considered if there are issues with infertility.
Adrenal insufficiency is also associated with radiation therapy to the cranium. A higher radiation dose or surgery to the suprasellar region increases the risk of adrenal insufficiency. A prior history of another hypothalamic/pituitary disorder also increases the risk of an adrenal insufficiency. Symptoms include lethargy, hypotension, hypoglycemia, anorexia, dehydration and failure to thrive. Symptoms should be assessed annually. Serum cortisol levels should be performed yearly for at least 15 years after treatment and as clinically indicated. Cortisol replacement should be considered as well as stress dosing as appropriate. An endocrinology consult should be considered if clinical suspicion is high for any of the above mentioned endocrine disorders.