Module 12: Simulation, Positioning, Verification and Immobilization
This content is no longer being maintained or updated. While it is available for review, please keep in mind the dates of creation, since some information may no longer be current.
For up-to-date proton therapy education designed for healthcare providers, please visit the Penn Radiation Medicine Institute. Current training and educational materials are available through our partnered and purchased programs.
encontrar mi
Simulation
Simulations for proton therapy are similar to photon-based simulations in that they are predominantly done using CT scanners at present. However, proton simulation does have a number of factors which require special consideration. Due to the greater conformality that protons allow, their limited range, and their dependence on tissue density, it is critical during CT simulation to take into account tissue motion. Any movement, such as ribs moving in and out of the treatment field, will affect the range of the protons. One way to account for this motion is to use 4D CT planning, which allows the physician to plan volumes around the entire path of tumor motion, hence allowing time to be factored in to the equation (i.e. the fourth dimension). This permits the physician to account for variations in the tumor size, shape, and position as it moves, and this volume is known as the internal margin. The combination of the clinical tumor volume (CTV) and the internal margin together forms the internal target volume or ITV. Using 4D CT to define an ITV is particularly useful in tumors with significant movement, such as lung cancers and upper abdominal tumors. Adequate immobilization is another way to limit patient motion during simulation and will be discussed in further detail below.
Protons range is greatly affected by tissue inhomogeneity, and thus this factor must be considered. Differences in tissue density affect the proton's range, and hence changes in density could affect coverage of the distal edge of the tumor. In order to account for this, the Hounsfield units from a CT scan must be converted to a water-equivalent density equivalent. This conversion is not perfect and can lead to a 1-2% error in calculating the effective range of a proton beam. Due to this uncertainty in range, many centers avoid placing critical structures, such as the spinal cord, at the end of the beam path. Often times, the lateral aspect of a proton beam is used to abut critical structure to avoid this situation. Adaptive treatment planning, where CT simulation is repeated during the course of treatment, may be necessary to account for the change in tumor density and size, as well as changes in patient weight.
Positioning: Robotic Treatment Couches and Chairs
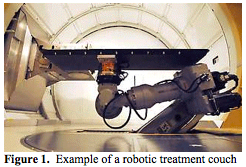
Precise patient positioning is a crucial aspect of proton therapy. The location of the distal edge of the proton beam is dependent on the density and the amount of tissue traversed, and hence precise interfractional patient positioning is vital. Additionally, to take full advantage of the greater conformality that protons allow, smaller margins may be used. Hence, precise positioning is needed to ensure that the target is appropriately covered in a consistent fashion to avoid marginal misses. Gantry based proton delivery allows the beam to be delivered from any angle within a single plain (360 degrees of rotation) and with a robotic based treatment couch, nearly any desired beam angle can be achieved. Proton beam tables are similar to photon tables in that they have movement in three dimensions as well as the ability to rotate about isocenter. However, in addition to these movements, robotic- based treatment couches also have pitch and roll capabilities that enable greater versatility in patient positioning. Robotic treatment couches may also allow greater accuracy and speed in positioning a patient compared with photon based treatment which use lasers alone for patient alignment.
Robotic based couches (Figure 1. Bloomington Indiana) can also be used in inclined beam systems. Though they only have two beam angles from which they can deliver protons (0 and 30 degrees from the vertical), the added flexibility of the robotic treatment couch still allows a large variety of treatment angles to be used.
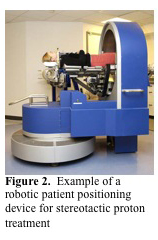
When a fixed beam is used, a traditional treatment couch does not allow different treatment angles to be used. However, there are a number of robotic patient positioning devices used in fixed proton therapy, such as the Stereotactic Alignment in Radiosurgery (STAR) device (figure 2. MGH). It is used for stereotactic proton therapy and allows the use of multiple beam angles. The couch is mounted in the center of a circle and can be moved, to a limited degree, in the lateral, vertical, and longitudinal directions. Additionally, the couch can be rotated to a limited degree in two axes about the isocenter. An example of a chair that is used with fixed beam proton radiosurgery is shown in (figure 3. MGH). They are generally designed to rotate a full 360 degrees in the vertical axis. The chair is also able to move in three axes (front to back, side to side and up and down) and can rotate about these axes to a limited degree. The utility of the chair is somewhat limited by the immobilization devices that can be used with it. Primarily head and neck cancers and cancers of the eye can be treated using the chair.
There are several important aspects to consider when using robotic tables. First, the majority of these devices are based on robotic arms designed for industrial use which places a premium on rapid acceleration and speed. For patient treatment, patient comfort and safety are tantamount. Thus, limits on speed, acceleration, angles, vibration, noise, along with a certain level of aesthetics, are necessary for patient comfort and safety. The majority of these limits can be achieved with modifications to the software driving the robotic arm. This being said, the table must also be designed such that the patient can be positioned on the table, immobilized, and then moved into treatment position in a relatively timely fashion. Additionally, manual control of the table in the event of power loss must also be ensured for patient safety. The accuracy of the table movements must also be precise (within 0.5 to 1 mm and within 0.3 degrees) and reproducible. The table must also be small enough to allow imaging devices such as cone beam CT to be utilized as well as anesthesia in patients who may require sedation for treatment. It is also important to note that any beam angles which must travel through the table must also account for the table's affect on proton range and scatter as this will affect the coverage of the tumor.
Verification Systems: Orthogonal X-rays, Markers, and Cone Beam CT
As stated above, as greater conformality is achieved with the use of protons, patient positioning becomes increasingly important. However, in order to take full advantage of a robotic treatment couch, accurate imaging of the tumor is crucial. Interfractional variability in patient positioning can be affected by daily setup error, changes in patient weight, and changes in tumor size. Proton range is affected by all of these factors as well as by changes in the density of the tissue the beam travels through. Hence if a bony structure moves into the treatment field due to setup error (when it was absent during treatment planning) or if the density of the tumor changes, this could affect the location of the distal edge of the proton field. There are a number of technologies which have been developed to optimize patient setup verification to minimize interfractional patient positioning variability.
Orthogonal X-rays depend on the patient's bony anatomy, and the tumors position relative to the bony anatomy, for reproducible positioning. Generally, a beam line view is achieved using a retractable X-ray tube in the nozzle of the proton unit. A retractable X-ray detector is used to capture the image from this X-ray tube. A second detector is located at 90 degrees to the patient with a separate X-ray source to get the lateral film. Prior to treatment, a pair of orthogonal diagnostic X-rays are taken and adjustments in patient positioning are made based on a comparison of these films to digitally reconstructed radiographs (DRR) generated from the initial treatment planning CT. At present there are a number of commercial packages, such as those by MedCom, which match the portal images to the DRR's and provide the shift information automatically for rapid setup and verification. There are several proton centers which use orthogonal imaging. However, there are several limitations to this technique. First, the tumor can move with respect to the bony anatomy, particularly in the thorax with respiratory motion and in the abdomen due to respiratory motion, bowel gas, stool and bladder fullness. Hence, even with orthogonal X-ray imaging, margins to account for this motion must be added unless fiducial markers are implanted within the tumor. Additionally, small rotational changes may be difficult to find on orthogonal films.
The difficulty with fiducial markers is that they affect heavy particle dose distribution. The effect of such a disturbance in dose distribution is dependent on the material from which the fiducial is made of, the size of the fiducial, the number of fiducials, and the orientation of the fiducial with respect to the beam. The distance the fiducial is from the distal edge of the beam also affects how much of a dose disturbance occurs secondary to the fiducial. Studies have suggested that, on average, the dose perturbation with stainless steel fiducials in the treatment of prostate cancer is about 5%. Recently, electromagnetic transponders have been used as fiducials (Calypso) which allow real time tracking of tumors to aid in both setup and intrafraction motion control. Studies to understand the compatibility of the Calypso system in the proton environment are ongoing. Several other companies are working to make fiducials which are compatible with proton therapy.
Cone beam CT scanning can accurately detect the position of soft tissues, such as the tumor, without relying on bony anatomy or fiducials. These are currently used in the photon environment and there is intense interest to develop a cone beam CT that will function in the proton setting. KV cone beam CT's deliver a similar dose to conventional CT's. However, it is important to note that the use of daily KV cone beam CT imaging for setup does appear to contribute a significant dose to normal tissues (2 cGy to 50 cGy for 20 treatments, depending on the anatomical site). MV cone beam CT contributes even more dose (2-3 times that of a KV CT scanner) but may be useful in patients who have high Z prostheses, such as hip replacements. Cone beam CT can be used for daily setup and a number of algorithms have been developed to match the treatment planning CT to the cone beam CT to optimize patient positioning. Studies of these algorithms have suggested that they are accurate to approximately 1 mm and 1 degree of rotation. Critical to this, is the precise alignment of the cone beam CT isocenter and the treatment isocenter. Due to mechanical flex in the treatment machine, there can be slight misalignments based on the angle of the gantry and models have been developed to account for this flex. Cone beam CT's may also play a role in treatment planning. If there is a major change in tumor volume, replanning off of the cone beam CT may be possible. However, cone beam CT's suffer from a poor estimation of the Hounsfield units of structures due to the scatter from the wide field used, lag in detector readout, and the limited collimation available during image acquisition by a cone beam CT. These factors can lead to approximately a 10% error in the estimated Hounsfield unit values. In proton therapy this is of particular concern as these estimates may affect the predicted range of the protons and the location of the distal edge of the beam.
Field Verification and In-situ PET Imaging
Electronic portal imaging has been a mainstay for field verification in photon based therapy. However, with proton based therapy, the protons have a limited range without exit dose and electronic portal imaging is potentially possible only in the setting of treating a body structure where the thickness is less then the proton range. This is called proton radiography and is currently under investigation but not currently ready for routing clinical use. Alternative means for the verification of dose distribution are needed in proton therapy. One interesting possibility is to take advantage of the induced radioactivity caused by protons within patients. Protons can cause the formation of isotopes with non-elastic collisions with nuclei. Some of the isotopes formed are positron emitters, which can then annihilate after combining with an electron and the resulting gamma rays can be detected by a PET scanner. This could allow in situ verification of the dose distribution. Several groups have investigated PET scanning both during the proton treatment itself as well as PET scanning after the treatment is complete. However, some of the positrons generated have a very short half life and rapid scanning is needed to capture as much information as possible. PET imaging can provide important spatial information regarding the distal and lateral edges of the treatment field. However, the limitations of the PET's resolution can limit how accurate the measures of these edges are. Regarding dose, the measured positron activity does not directly represent the dose delivered; nevertheless, the amount of activity expected can be predicted and compared to the measured PET activity. Any deviation seen between the measured and predicted activity suggests a dosing error.
Immobilization
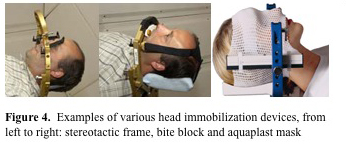
The main goal of patient immobilization is to minimize the patient's motion during treatment as well as facilitate rapid and consistent setup between fractions (which depends on the reproducibility of the patient position within the immobilization device and the immobilization devices position with respect to the treatment beams). In proton therapy, the distance from the patient surface to the tumor is also an important factor and this may require more rigid immobilization to maintain the proper shape of the patient. Much like in photon therapy, the immobilization devices should be easily customizable for the specific dimensions of the patient and easily transportable from the imaging area to the treatment area. There are several major differences between immobilization devices that are used with protons versus those used with photon therapy. One important difference with protons is that there is no build up, and hence there is no bolus effect of the immobilization device on the patient's skin. Therefore patient immobilization devices which are in direct contact with the skin which is within the field of the beam can be used. However, the proton range is highly dependent on the amount of material traversed (due to energy loss) and this extra material must be accounted for. It is also important to note that immobilizations devices within the proton field can influence the lateral edge of the beam, with thicker materials creating a greater increase in the lateral penumbra. To minimize both of these effects the thickness of the immobilization device within the field should be kept to a minimum and the material should be as homogenous as possible. For head and neck and CNS tumors a number of immobilization devices have been used with protons including aquaplast masks, bite blocks, and stereotactic frames (figure 4. MGH and Varian).
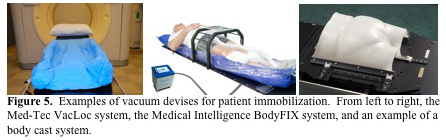
There are also several immobilization devices used for thoracic and pelvic radiation therapy. There are several vacuum devices that are available for patient immobilization such as the Med-Tec VacLoc system and the Medical Intelligence BodyFIX system (Figure 5. MGH).
The majority of these devices have been designed for other medical applications and have been adapted for use with proton therapy. As mentioned above, extensive testing should be performed to determine how much the additional material used in the immobilization devise affects the proton beam range and lateral penumbra, and these factors must be accounted for during treatment planning.