Instituto Nacional del Cáncer
Fecha de publicación: Apr 4, 2024
Información sobre las opciones de tratamiento del carcinoma de nasofaringe recién diagnosticado y recidivante, como radioterapia, quimiorradioterapia seguida de quimioterapia adyuvante, cirugía y quimioterapia. Resumen para profesionales de la salud.
Tratamiento del carcinoma de nasofaringe
Información general sobre el carcinoma de nasofaringe
En la nasofaringe se presentan tumores de muchos tipos histológicos diferentes, pero en este resumen solo se tratan los carcinomas de nasofaringe. Los estadios del American Joint Committee on Cancer hacen referencia solo al carcinoma de nasofaringe de grado I, II y III de la clasificación de la Organización Mundial de la Salud (OMS).
Incidencia y mortalidad
Menos de 1 persona por cada 100 000 reciben un diagnóstico de carcinoma de nasofaringe en el mundo cada año; la mayor cantidad de casos se presentan en el sur de China, el Sudeste asiático, el ártico, así como el Oriente medio y el norte de África. La incidencia es más alta en hombres que en mujeres. El carcinoma de nasofaringe de grado I de la OMS (subtipo queratinizante) representa menos del 20 % de los casos en los Estados Unidos, mientras que los grados II y III constituyen la forma endémica de carcinoma de nasofaringe y se encuentran sobre todo en Asia. Los subtipos no queratinizantes se relacionan con la infección por el virus de Epstein-Barr (VEB) y constituyen la mayoría de los casos.
Características anatómicas
La nasofaringe tiene una forma cuboide. Las paredes laterales están formadas por la trompa de Eustaquio y la fosa de Rosenmüller. El techo, inclinado en sentido anteroposterior, está delimitado por la hipófisis faríngea, la amígdala faríngea, la bolsa faríngea, y en la parte superior por la base del cráneo. La porción anterior de la nasofaringe colinda con la coana posterior y la cavidad nasal, y la porción posterior está demarcada por los músculos de la pared faríngea posterior. La porción inferior de la nasofaringe se delimita con una línea horizontal imaginaria formada por la superficie superior del paladar blando y la pared faríngea posterior. El carcinoma de nasofaringe se origina en las células epiteliales que revisten la nasofaringe.
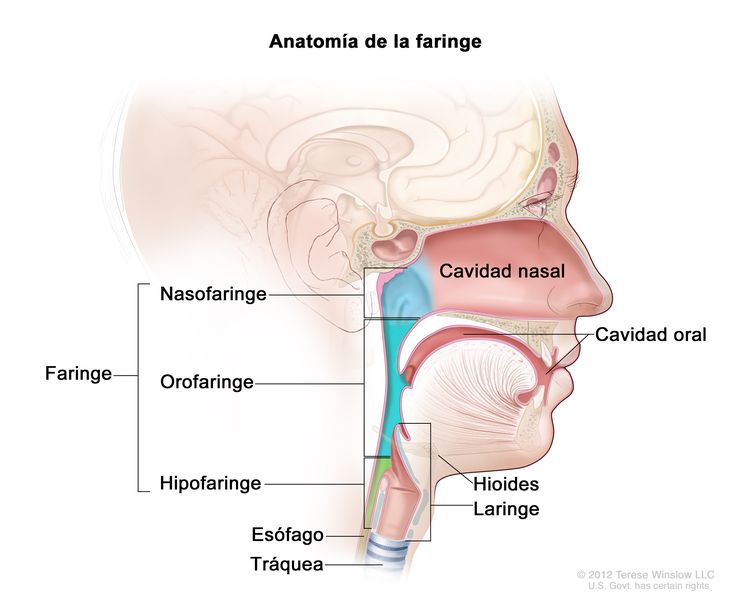 Anatomía de la faringe.
Anatomía de la faringe.
Factores de riesgo
Los factores de riesgo del carcinoma de nasofaringe son los siguientes.
- Ingesta excesiva de bebidas alcohólicas.
- Antecedentes de consumo de cigarrillos.
- Raza asiática.
- Exposición al VEB.
- Antecedentes familiares.
Características clínicas
Los signos y síntomas del cuadro clínico inicial son los siguientes:
- Cefalea causada por disfunción de los pares craneales (por lo general, II–VI o IX–XII).
- Diplopía.
- Adormecimiento de la cara.
- Adenopatía cervical (presente en cerca del 75 % de los pacientes, a menudo, bilateral y posterior).
- Obstrucción nasal.
- Epistaxis.
- Hipoacusia.
- Acúfenos.
- Otitis media.
- Dolor de garganta.
En los pacientes que presentan adenopatía cervical sola, el hallazgo tisular de material genómico del VEB, mediante reacción en cadena de la polimerasa (PCR), es un indicio fuerte de un tumor primario de nasofaringe y esa área se debe examinar de manera detallada.
Evaluación diagnóstica
Pruebas y procedimientos diagnósticos
El diagnóstico se establece mediante biopsia de la masa nasofaríngea. Las siguientes pruebas y procedimientos se usan para el diagnóstico del carcinoma de nasofaringe:
- Exploración visual detallada mediante endoscopia nasal con fibra óptica o exploración con anestesia.
- Biopsia endoscópica.
- Examen físico y antecedentes de salud. Documentación del tamaño y la ubicación del tumor, y de los ganglios linfáticos cervicales.
- Evaluación del funcionamiento de los pares craneales que incluya un examen neuroftalmológico y auditivo.
- Tomografía computarizada (TC) o tomografía por emisión de positrones con tomografía computarizada (TEP-TC).
- Imágenes por resonancia magnética (IRM) para evaluar la invasión a la base del cráneo.
- DNA del VEB circulante en plasma que proviene de células cancerosas.
- Prueba sanguínea del virus del papiloma humano (VPH) de tipo 16, si la prueba del VEB fuera negativa.
Cualquier hallazgo clínico o de laboratorio que indique metástasis a distancia conlleva una evaluación adicional de otros sitios. La IRM suele ser más útil que la TC para evaluar el compromiso de la base del cráneo y definir la extensión de las anomalías detectadas.
DNA del VEB circulante que proviene de células cancerosas
El DNA del VEB en muestras de plasma de poblaciones endémicas en ocasiones es útil como examen de detección del carcinoma de nasofaringe asintomático en un estadio temprano. El DNA del VEB circulante en plasma que proviene de células cancerosas es un marcador tumoral establecido para el carcinoma de nasofaringe; tiene una sensibilidad del 96 % y una especificidad del 93 %. La presencia de fragmentos cortos de DNA del VEB con menos de 181 pares de bases en el plasma de los pacientes con carcinoma de nasofaringe indica que las moléculas de DNA del VEB pasan a la circulación sanguínea por apoptosis de las células cancerígenas en lugar de replicación vírica activa.
Evidencia (DNA del VEB en plasma como examen de detección y diagnóstico del carcinoma de nasofaringe):
- En un estudio con 20 174 participantes en China se usó el DNA del VEB en plasma para la detección temprana del carcinoma de nasofaringe.
- Al comienzo del estudio, la prueba para DNA del VEB en plasma fue positiva en 1112 participantes.
- De los pacientes, 309 (1,5 % de todos los participantes y 27,8 % de los que tuvieron un resultado positivo en un principio) presentaron DNA del VEB en plasma detectable de manera persistente en la valoración inicial y durante el seguimiento.
- Entre los 309 participantes, el carcinoma de nasofaringe se confirmó después de un examen endoscópico nasal, IRM y biopsia en 34 pacientes (11,0 %).
Virus del papiloma humano
La identificación del carcinoma de nasofaringe relacionado con el virus del papiloma humano (VPH) requiere la detección de p16 mediante tinción inmunohistoquímica, hibridación in situ o reacción en cadena de la polimerasa, de una manera similar a la forma en que se identifica el cáncer de orofaringe relacionado con el VPH. Menos del 10 % de los carcinomas de nasofaringe no queratinizantes están relacionados con la infección por el VPH.
Factores pronósticos
Otros factores pronósticos que quizás afecten el resultados del tratamiento son los siguientes:
- Tumor de grado I de la OMS
- Estadio de tumor (T) más alto.
- Compromiso ganglionar (N) cervical.
- Concentraciones altas de DNA del VEB en plasma o suero antes del tratamiento y después de este.
- Tamaño grande del tumor.[Nivel de evidencia C1]
Pruebas de seguimiento y efectos tardíos
El seguimiento de la recidiva del tumor incluye las siguientes pruebas:
- Examen periódico de rutina del lugar donde se originó el tumor y el cuello.
- TC o TEP-TC.
- IRM.
- Concentraciones de DNA del VEB en plasma o suero.
Se debe hacer un seguimiento de los pacientes para detectar posibles efectos tardíos del seguimiento como los siguientes:
- Xerostomía.
- Complicaciones dentales y orales.
- Hipoacusia.
- Pérdida de visión.
- Disfagia.
- Trismo.
- Problemas en el funcionamiento de la tiroides y la hipófisis.
- Neuropatías craneales.
- Deterioro cognitivo.
Aunque la mayoría de las recidivas se presentan dentro de los primeros 5 años desde el diagnóstico, también pueden ocurrir luego de intervalos más largos. La incidencia de segundos cánceres primarios después del tratamiento es menor para el carcinoma de nasofaringe que para otros cánceres de cabeza y cuello.
A partir de la evidencia acumulada se observó una alta incidencia (>30–40 %) de hipotiroidismo en pacientes que recibieron radioterapia de haz externo (RTE) dirigida a toda la glándula tiroidea o a la hipófisis. Se debería considerar la evaluación del funcionamiento de la tiroides en los pacientes antes de la terapia y como parte del seguimiento después del tratamiento.
Antes de iniciar la radioterapia, es muy importante la evaluación minuciosa de la higiene dental y oral, y su tratamiento. La radioterapia de intensidad modulada (RTIM) produce una incidencia más baja de xerostomía y quizás se traduzca en una mejor calidad de vida que la radioterapia convencional tridimensional o bidimensional (RT2D).[Nivel de evidencia A3]
Evidencia (RTIM vs. RT2D y la incidencia de xerostomía)
- En un estudio prospectivo aleatorizado se evaluó la incidencia de xerostomía en pacientes con carcinoma de nasofaringe en estadio temprano que se trataron con RTIM (n = 28) o RT2D (n = 28). Se determinó el grado de toxicidad a largo plazo de acuerdo a los criterios del Radiation Therapy Oncology Group (RTOG).
- La incidencia de xerostomía de grado 2 fue del 20 % en los pacientes que recibieron RTIM y del 90 % en los pacientes que recibieron RT2D (P = 0,001). No se observaron diferencias significativas entre los grupos en los cuestionarios de xerostomía.
- Los pacientes que recibieron RTIM tuvieron puntajes más bajos de sequedad en la boca que los pacientes que recibieron RT2D.
- La tasa de supervivencia general fue del 82 % en el grupo de RTIM versus el 54 % en el grupo de RT2D.
- La tasa de supervivencia sin recaída fue del 70 % en el grupo de RTIM versus el 54 % en el grupo de RT2D.
- Se notificaron más complicaciones tardías entre los pacientes del grupo de RT2D.
- En un estudio de fase II del Radiation Therapy Oncology Group (RTOG) (RTOG-0225) se demostró la factibilidad de la RTIM en un entorno multicéntrico.
- La tasa de xerostomía de grado 2 a 1 año desde del comienzo de la RTIM fue del 13,5 %.
- La tasa de xerostomía de grado 3 y 4 fue mínima.
- Solo 2 de 68 pacientes notificaron xerostomía de grado 3.
- Ninguno de los pacientes presentó xerostomía de grado 4.
References
- Petersson F: Nasopharyngeal carcinoma: a review. Semin Diagn Pathol 32 (1): 54-73, 2015.
- Ferlay J, Soerjomataram I, Dikshit R, et al.: Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136 (5): E359-86, 2015.
- Chang ET, Adami HO: The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15 (10): 1765-77, 2006.
- Chen YP, Chan ATC, Le QT, et al.: Nasopharyngeal carcinoma. Lancet 394 (10192): 64-80, 2019.
- Chien YC, Chen JY, Liu MY, et al.: Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 345 (26): 1877-82, 2001.
- Chen L, Gallicchio L, Boyd-Lindsley K, et al.: Alcohol consumption and the risk of nasopharyngeal carcinoma: a systematic review. Nutr Cancer 61 (1): 1-15, 2009.
- Okekpa SI, S M N Mydin RB, Mangantig E, et al.: Nasopharyngeal Carcinoma (NPC) Risk Factors: A Systematic Review and Meta-Analysis of the Association with Lifestyle, Diets, Socioeconomic and Sociodemographic in Asian Region. Asian Pac J Cancer Prev 20 (11): 3505-3514, 2019.
- Xie SH, Yu IT, Tse LA, et al.: Tobacco smoking, family history, and the risk of nasopharyngeal carcinoma: a case-referent study in Hong Kong Chinese. Cancer Causes Control 26 (6): 913-21, 2015.
- Feinmesser R, Miyazaki I, Cheung R, et al.: Diagnosis of nasopharyngeal carcinoma by DNA amplification of tissue obtained by fine-needle aspiration. N Engl J Med 326 (1): 17-21, 1992.
- Cummings CW, Fredrickson JM, Harker LA, et al.: Otolaryngology - Head and Neck Surgery. Mosby-Year Book, Inc., 1998.
- Kim KY, Le QT, Yom SS, et al.: Clinical Utility of Epstein-Barr Virus DNA Testing in the Treatment of Nasopharyngeal Carcinoma Patients. Int J Radiat Oncol Biol Phys 98 (5): 996-1001, 2017.
- Mendenhall WM, Werning JW, Pfister DG: Treatment of head and neck cancer. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 729-80.
- Laramore GE, ed.: Radiation Therapy of Head and Neck Cancer. Springer-Verlag, 1989.
- Chan KCA, Woo JKS, King A, et al.: Analysis of Plasma Epstein-Barr Virus DNA to Screen for Nasopharyngeal Cancer. N Engl J Med 377 (6): 513-522, 2017.
- Lo YM, Chan LY, Lo KW, et al.: Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res 59 (6): 1188-91, 1999.
- Leung SF, Zee B, Ma BB, et al.: Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 24 (34): 5414-8, 2006.
- Chan KC, Zhang J, Chan AT, et al.: Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res 63 (9): 2028-32, 2003.
- Huang WB, Chan JYW, Liu DL: Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: Multicenter study from an endemic area in Southern China. Cancer 124 (3): 530-536, 2018.
- Robinson M, Suh YE, Paleri V, et al.: Oncogenic human papillomavirus-associated nasopharyngeal carcinoma: an observational study of correlation with ethnicity, histological subtype and outcome in a UK population. Infect Agent Cancer 8 (1): 30, 2013.
- Sanguineti G, Geara FB, Garden AS, et al.: Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of local and regional control. Int J Radiat Oncol Biol Phys 37 (5): 985-96, 1997.
- Leung SF, Chan AT, Zee B, et al.: Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer 98 (2): 288-91, 2003.
- Chan AT, Lo YM, Zee B, et al.: Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst 94 (21): 1614-9, 2002.
- Lee CC, Huang TT, Lee MS, et al.: Clinical application of tumor volume in advanced nasopharyngeal carcinoma to predict outcome. Radiat Oncol 5: 20, 2010.
- Cooper JS, Fu K, Marks J, et al.: Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys 31 (5): 1141-64, 1995.
- McDowell L, Corry J, Ringash J, et al.: Quality of Life, Toxicity and Unmet Needs in Nasopharyngeal Cancer Survivors. Front Oncol 10: 930, 2020.
- Fong R, Ward EC, Rumbach AF: Dysphagia after chemo-radiation for nasopharyngeal cancer: A scoping review. World J Otorhinolaryngol Head Neck Surg 6 (1): 10-24, 2020.
- Cooper JS, Scott C, Marcial V, et al.: The relationship of nasopharyngeal carcinomas and second independent malignancies based on the Radiation Therapy Oncology Group experience. Cancer 67 (6): 1673-7, 1991.
- Turner SL, Tiver KW, Boyages SC: Thyroid dysfunction following radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 31 (2): 279-83, 1995.
- Constine LS: What else don't we know about the late effects of radiation in patients treated for head and neck cancer? Int J Radiat Oncol Biol Phys 31 (2): 427-9, 1995.
- Pow EH, Kwong DL, McMillan AS, et al.: Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys 66 (4): 981-91, 2006.
- Kam MK, Leung SF, Zee B, et al.: Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 25 (31): 4873-9, 2007.
- Poon DMC, Kam MKM, Johnson D, et al.: Durability of the parotid-sparing effect of intensity-modulated radiotherapy (IMRT) in early stage nasopharyngeal carcinoma: A 15-year follow-up of a randomized prospective study of IMRT versus two-dimensional radiotherapy. Head Neck 43 (6): 1711-1720, 2021.
- Lee N, Harris J, Garden AS, et al.: Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 27 (22): 3684-90, 2009.
Clasificación celular del carcinoma de nasofaringe
La Organización Mundial de la Salud (OMS) define el carcinoma de nasofaringe como un “carcinoma que se forma en la mucosa nasofaríngea y que exhibe indicios microscópicos o ultraestructurales de diferenciación escamosa”. La clasificación de la OMS para el carcinoma de nasofaringe ha evolucionado con el tiempo y la clasificación de 2005 es la vigente. Se usan las tres versiones descritas a continuación. Las definiciones de 1978 se siguen usando, en particular para los carcinomas indiferenciados que tienen el pronóstico más precario y la mayor sensibilidad a la quimiorradioterapia.
Clasificación de la OMS de 1978:
- Carcinoma de células escamosas.
- Carcinoma de células escamosas no queratinizantes.
- Carcinoma indiferenciado (subtipo más común).
Clasificación de la OMS de 1991:
- Carcinoma de células escamosas.
- Carcinoma de células escamosas no queratinizantes.
- Carcinoma no queratinizante diferenciado.
- Carcinoma indiferenciado.
Clasificación de la OMS de 2005:
- Carcinoma de células escamosas queratinizantes.
- Carcinoma no queratinizante.
- Carcinoma no queratinizante diferenciado.
- Carcinoma indiferenciado.
- Carcinoma de células escamosas basaloides.
Entre las subdivisiones previas del carcinoma de nasofaringe se incluía el linfoepitelioma, que ahora se clasifica como un carcinoma de grado III de la OMS y se caracteriza por infiltrado linfoide.
References
- Thompson LD: Update on nasopharyngeal carcinoma. Head Neck Pathol 1 (1): 81-6, 2007.
- Wang HY, Chang YL, To KF, et al.: A new prognostic histopathologic classification of nasopharyngeal carcinoma. Chin J Cancer 35: 41, 2016.
- Stelow EB, Wenig BM: Update From The 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Nasopharynx. Head Neck Pathol 11 (1): 16-22, 2017.
- Shanmugaratnam K, Chan SH, de-Thé G, et al.: Histopathology of nasopharyngeal carcinoma: correlations with epidemiology, survival rates and other biological characteristics. Cancer 44 (3): 1029-44, 1979.
- Shanmugaratnam K, Sobin L: Histological Typing of Upper Respiratory Tract Tumours. World Health Organization, 1978. International Histologic Classification of Tumours: No. 19.
Información sobre los estadios del carcinoma de nasofaringe
Los sistemas de estadificación se usan para la estadificación clínica, y se hacen teniendo en cuenta la mejor estimación posible de la extensión de la enfermedad antes del tratamiento.
La evaluación del tumor primario se hace a partir de la inspección, palpación y evaluación endoscópica con fibra óptica. Se debe confirmar el tumor mediante un estudio histológico e incluir cualquier otro dato patológico obtenido en la biopsia. La evaluación del funcionamiento de los pares craneales es importante en los tumores de nasofaringe. Las áreas de drenaje ganglionar se examinan mediante palpación cuidadosa y evaluación radiológica. Los ganglios linfáticos retrofaríngeos son el primer escalón de drenaje.
La información de los siguientes estudios de diagnóstico por imagen se usa para la estadificación:
- Las imágenes por resonancia magnética brindan información adicional a la tomografía computarizada (TC) en la evaluación de la invasión a la base del cráneo y la diseminación intracraneal.
- La tomografía por emisión de positrones en combinación con la TC son útiles en la planificación de la radioterapia para la demarcación del tumor primario y ayudan a detectar el compromiso metastásico ganglionar o la diseminación metastásica, por ejemplo, pulmonar o esquelética en pacientes con carcinoma de nasofaringe avanzado.
Si el paciente sufre una recaída, deberá hacerse una nueva evaluación completa para seleccionar la terapia adicional apropiada.
Agrupamiento por estadios y definiciones TNM del American Joint Committee on Cancer
El American Joint Committee on Cancer (AJCC) designó los estadios mediante la clasificación TNM (tumor, ganglio linfático y metástasis) para definir el carcinoma de nasofaringe.
| Estadio | TNM | Descripción |
|---|---|---|
| T = tumor primario; N = ganglio linfático regional; M = metástasis a distancia. | ||
| aReproducción autorizada de AJCC: Nasopharynx. En: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8.ª edición Nueva York, NY: Springer, 2017, págs. 103-11. | ||
| 0 | Tis, N0, M0 | Tis = carcinoma in situ. |
| N0 = sin metástasis en ganglios linfáticos regionales. | ||
| M0 = sin metástasis a distancia. | ||
| Estadio | TNM | Descripción |
|---|---|---|
| T = tumor primario; N = ganglio linfático regional; M = metástasis a distancia. | ||
| aReproducción autorizada de AJCC: Nasopharynx. En: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8.ª edición Nueva York, NY: Springer, 2017, págs. 103-11. | ||
| I | T1, N0, M0 | T1 = tumor limitado a la nasofaringe, o diseminación a la orofaringe o la cavidad nasal sin compromiso parafaríngeo. |
| N0 = sin metástasis en ganglios linfáticos regionales. | ||
| M0 = sin metástasis a distancia. | ||
| Estadio | TNM | Descripción |
|---|---|---|
| T = tumor primario; N = ganglio linfático regional; M = metástasis a distancia; VEB = virus de Epstein-Barr. | ||
| aReproducción autorizada de AJCC: Nasopharynx. En: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8.ª edición Nueva York, NY: Springer, 2017, págs. 103-11. | ||
| II | T0, Tis, T1, N1, M0 | T0 = no se identifica un tumor, pero hay compromiso de uno o más ganglios cervicales positivos para VEB. |
| Tis = carcinoma in situ. | ||
| T1 = tumor limitado a la nasofaringe o con diseminación a la orofaringe o la cavidad nasal sin compromiso parafaríngeo. | ||
| N1 = metástasis unilateral en uno o más ganglios linfáticos cervicales, o metástasis unilaterales o bilaterales en uno o más ganglios linfáticos retrofaríngeos que miden ≤6 cm en su mayor dimensión y se ubican por encima del borde inferior del cartílago cricoides. | ||
| M0 = sin metástasis a distancia. | ||
| T2, N0, M0 | T2 = tumor con diseminación al espacio parafaríngeo o compromiso de tejidos blandos adyacentes (músculos pterigoideo medial, pterigoideo lateral y prevertebrales). | |
| N0 = sin metástasis en ganglios linfáticos regionales. | ||
| M0 = sin metástasis a distancia. | ||
| T2, N1, M0 | T2 = tumor con diseminación al espacio parafaríngeo o compromiso de tejidos blandos adyacentes (músculos pterigoideo medial, pterigoideo lateral y prevertebrales). | |
| N1 = metástasis unilateral en uno o más ganglios linfáticos cervicales, o metástasis unilaterales o bilaterales en uno o más ganglios linfáticos retrofaríngeos que miden ≤6 cm en su mayor dimensión y se ubican por encima del borde inferior del cartílago cricoides. | ||
| M0 = sin metástasis a distancia. | ||
| Estadio | TNM | Descripción |
|---|---|---|
| T = tumor primario; N = ganglio linfático regional; M = metástasis a distancia; VEB = virus de Epstein-Barr. | ||
| aReproducción autorizada de AJCC: Nasopharynx. En: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8.ª edición Nueva York, NY: Springer, 2017, págs. 103-11. | ||
| III | T0, Tis, T1, N2, M0 | T0 = no se identifica un tumor, pero hay compromiso de uno o más ganglios cervicales positivos para VEB. |
| Tis = carcinoma in situ. | ||
| T1 = tumor limitado a la nasofaringe o con diseminación a la orofaringe o la cavidad nasal sin compromiso parafaríngeo. | ||
| N2 = metástasis bilaterales en uno o más ganglios linfáticos cervicales que miden ≤6 cm en su mayor dimensión y se ubican por encima del borde inferior del cartílago cricoides. | ||
| M0 = sin metástasis a distancia. | ||
| T2, N2, M0 | T2 = tumor con diseminación al espacio parafaríngeo o compromiso de tejidos blandos adyacentes (músculos pterigoideo medial, pterigoideo lateral y prevertebrales). | |
| N2 = metástasis bilaterales en uno o más ganglios linfáticos cervicales que miden ≤6 cm en su mayor dimensión y se ubican por encima del borde inferior del cartílago cricoides. | ||
| M0 = sin metástasis a distancia. | ||
| T3, N0, M0 | T3 = tumor con infiltración de estructuras óseas en la base del cráneo, vertebras cervicales, estructuras pterigoideas o senos paranasales. | |
| N0 = sin metástasis en ganglios linfáticos regionales. | ||
| M0 = sin metástasis a distancia. | ||
| T3, N1, M0 | T3 = tumor con infiltración de estructuras óseas en la base del cráneo, vertebras cervicales, estructuras pterigoideas o senos paranasales. | |
| N1 = metástasis unilateral en uno o más ganglios linfáticos cervicales, o metástasis unilaterales o bilaterales en uno o más ganglios linfáticos retrofaríngeos que miden ≤6 cm en su mayor dimensión y se ubican por encima del borde inferior del cartílago cricoides. | ||
| M0 = sin metástasis a distancia. | ||
| T3, N2, M0 | T3 = tumor con infiltración de estructuras óseas en la base del cráneo, vertebras cervicales, estructuras pterigoideas o senos paranasales. | |
| N2 = metástasis bilaterales en uno o más ganglios linfáticos cervicales que miden ≤6 cm en su mayor dimensión y se ubican por encima del borde inferior del cartílago cricoides. | ||
| M0 = sin metástasis a distancia. | ||
| Estadio | TNM | Descripción |
|---|---|---|
| T = tumor primario; N = ganglio linfático regional; M = metástasis a distancia; VEB = virus de Epstein-Barr. | ||
| aReproducción autorizada de AJCC: Nasopharynx. En: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8.ª edición Nueva York, NY: Springer, 2017, págs. 103-11. | ||
| IVA | T4, N0, M0 | T4 = tumor con diseminación intracraneal, compromiso de nervios craneales, hipofaringe, órbita, glándula paratiroidea o infiltración generalizada de tejidos blandos más allá de la superficie lateral del músculo pterigoideo lateral. |
| N0 = sin metástasis en ganglios linfáticos regionales. | ||
| M0 = sin metástasis a distancia. | ||
| T4, N1, M0 | T4 = tumor con diseminación intracraneal, compromiso de nervios craneales, hipofaringe, órbita, glándula paratiroidea o infiltración generalizada de tejidos blandos más allá de la superficie lateral del músculo pterigoideo lateral. | |
| N1 = metástasis unilateral en uno o más ganglios linfáticos cervicales, o metástasis unilaterales o bilaterales en uno o más ganglios linfáticos retrofaríngeos que miden ≤6 cm en su mayor dimensión y se ubican por encima del borde inferior del cartílago cricoides. | ||
| M0 = sin metástasis a distancia. | ||
| T4, N2, M0 | T4 = tumor con diseminación intracraneal, compromiso de nervios craneales, hipofaringe, órbita, glándula paratiroidea o infiltración generalizada de tejidos blandos más allá de la superficie lateral del músculo pterigoideo lateral. | |
| N2 = metástasis bilaterales en uno o más ganglios linfáticos cervicales que miden ≤6 cm en su mayor dimensión y se ubican por encima del borde inferior del cartílago cricoides. | ||
| M0 = sin metástasis a distancia. | ||
| Cualquier T, N3, M0 | TX = tumor primario no evaluable. | |
| T0 = no se identifica un tumor, pero hay compromiso de uno o más ganglios cervicales positivos para VEB. | ||
| Tis = carcinoma in situ. | ||
| T1 = tumor limitado a la nasofaringe o con diseminación a la orofaringe o la cavidad nasal sin compromiso parafaríngeo. | ||
| T2 = tumor con diseminación al espacio parafaríngeo o compromiso de tejidos blandos adyacentes (músculos pterigoideo medial, pterigoideo lateral y prevertebrales). | ||
| T3 = tumor con infiltración de estructuras óseas en la base del cráneo, vertebras cervicales, estructuras pterigoideas o senos paranasales. | ||
| T4 = tumor con diseminación intracraneal, compromiso de nervios craneales, hipofaringe, órbita, glándula paratiroidea o infiltración generalizada de tejidos blandos más allá de la superficie lateral del músculo pterigoideo lateral. | ||
| N3 = metástasis unilaterales o bilaterales en uno o más ganglios linfáticos cervicales que miden >6 cm en su mayor dimensión o diseminación por debajo del borde inferior del cartílago cricoides. | ||
| M0 = sin metástasis a distancia. | ||
| IVB | Cualquier T, cualquier N, M1 | Cualquier T = consultar la descripción del estadio IVA en este cuadro. |
| NX = ganglios linfáticos regionales no evaluables. | ||
| N0 = sin metástasis en ganglios linfáticos regionales. | ||
| N1 = metástasis unilateral en uno o más ganglios linfáticos cervicales, o metástasis unilaterales o bilaterales en uno o más ganglios linfáticos retrofaríngeos que miden ≤6 cm en su mayor dimensión y se ubican por encima del borde inferior del cartílago cricoides. | ||
| N2 = metástasis bilaterales en uno o más ganglios linfáticos cervicales que miden ≤6 cm en su mayor dimensión y se ubican por encima del borde inferior del cartílago cricoides. | ||
| N3 = metástasis unilaterales o bilaterales en uno o más ganglios linfáticos cervicales que miden >6 cm en su mayor dimensión o diseminación por debajo del borde inferior del cartílago cricoides. | ||
| M1 = metástasis a distancia. | ||
References
- Teo PM, Leung SF, Yu P, et al.: A comparison of Ho's, International Union Against Cancer, and American Joint Committee stage classifications for nasopharyngeal carcinoma. Cancer 67 (2): 434-9, 1991.
- Lee AW, Foo W, Law SC, et al.: Staging of nasopharyngeal carcinoma: from Ho's to the new UICC system. Int J Cancer 84 (2): 179-87, 1999.
- Mendenhall WM, Werning JW, Pfister DG: Treatment of head and neck cancer. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 729-80.
- Laramore GE, ed.: Radiation Therapy of Head and Neck Cancer. Springer-Verlag, 1989.
- Consensus conference. Magnetic resonance imaging. JAMA 259 (14): 2132-8, 1988.
- Liu FY, Chang JT, Wang HM, et al.: [18F]fluorodeoxyglucose positron emission tomography is more sensitive than skeletal scintigraphy for detecting bone metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 24 (4): 599-604, 2006.
- Nasopharynx. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 103–11.
Aspectos generales de las opciones de tratamiento del carcinoma de nasofaringe
| Estadio | Opciones de tratamiento |
|---|---|
| Carcinoma de nasofaringe en estadio I | Radioterapia |
| Carcinoma de nasofaringe en estadios II, III y IV | Radioterapia |
| Quimiorradioterapia simultánea | |
| Quimioterapia neoadyuvante y quimiorradioterapia simultánea | |
| Quimiorradioterapia simultánea y quimioterapia adyuvante | |
| Quimioterapia neoadyuvante seguida de radioterapia sola | |
| Carcinoma de nasofaringe recidivante | Radioterapia |
Administración de fluorouracilo
El gen de la dihidropirimidina–deshidrogenasa (DPYD), codifica la enzima que cataboliza las pirimidinas y las fluoropirimidinas, como la capecitabina y el fluorouracilo. Se estima que entre el 1 % y el 2 % de la población tiene variantes defectuosas de la enzima dihidropirimidina–deshidrogenasa DPYD que produce la reducción de la función de la proteína DPD y la acumulación de pirimidinas y fluoropirimidinas en el cuerpo. Los pacientes con la variante DPYD*2A que reciben fluoropirimidinas quizás presenten efectos tóxicos graves que ponen en riesgo la salud, y a veces son mortales. Se han identificado muchas otras variantes de DPYD, con diferentes efectos clínicos. Es posible que se recomiende evitar la fluoropirimidina o reducir la dosis al 50 % según el genotipo DPYD del paciente y el número de alelos funcionales de DPYD. Las pruebas genéticas para DPYD cuestan menos de $200, pero la cobertura del seguro varía debido a la falta de directrices nacionales. Además, es posible que las pruebas retrasen el tratamiento por 2 semanas, lo que no sería aconsejable en casos de urgencia. Este tema es objeto de controversia y requiere evaluación adicional.
References
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021.
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016.
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021.
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018.
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018.
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022.
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022.
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023.
Tratamiento del carcinoma de nasofaringe en estadio I
Opciones de tratamiento del carcinoma de nasofaringe en estadio I
Las opciones de tratamiento del carcinoma de nasofaringe en estadio I son las siguientes:
- Radioterapia.
Radioterapia
La radioterapia de dosis alta con quimioterapia es el tratamiento inicial para el carcinoma de nasofaringe. Se administra radioterapia de dosis alta en el sitio del tumor primario y radioterapia profiláctica en los ganglios linfáticos regionales bilaterales del cuello. La dosis de radioterapia y los márgenes del campo se ajustan de manera individual según la ubicación y el tamaño del tumor primario y los ganglios linfáticos.
La mayoría de los tumores se tratan solo con radioterapia de haz externo (RTE). En algunos pacientes, a veces se refuerza la radioterapia con implantes intracavitarios o intersticiales, o mediante el uso de radiocirugía estereotáctica cuando se cuenta con la pericia clínica y las características anatómicas son las adecuadas.
Ensayos clínicos en curso
Realizar una búsqueda avanzada en inglés de los ensayos clínicos sobre cáncer auspiciados por el NCI que ahora aceptan pacientes. La búsqueda se puede simplificar por ubicación del ensayo, tipo de tratamiento, nombre del fármaco y otros criterios. También se dispone de información general sobre los ensayos clínicos.
References
- Baujat B, Audry H, Bourhis J, et al.: Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 64 (1): 47-56, 2006.
- Xiao WW, Han F, Lu TX, et al.: Treatment outcomes after radiotherapy alone for patients with early-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 74 (4): 1070-6, 2009.
- Perez CA, Devineni VR, Marcial-Vega V, et al.: Carcinoma of the nasopharynx: factors affecting prognosis. Int J Radiat Oncol Biol Phys 23 (2): 271-80, 1992.
- Lee AW, Law SC, Foo W, et al.: Nasopharyngeal carcinoma: local control by megavoltage irradiation. Br J Radiol 66 (786): 528-36, 1993.
- Geara FB, Sanguineti G, Tucker SL, et al.: Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of distant metastasis and survival. Radiother Oncol 43 (1): 53-61, 1997.
- Sanguineti G, Geara FB, Garden AS, et al.: Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of local and regional control. Int J Radiat Oncol Biol Phys 37 (5): 985-96, 1997.
- Mendenhall WM, Werning JW, Pfister DG: Treatment of head and neck cancer. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 729-80.
- Itami J, Anzai Y, Nemoto K, et al.: Prognostic factors for local control in nasopharyngeal cancer (NPC): analysis by multivariate proportional hazard models. Radiother Oncol 21 (4): 233-9, 1991.
- Levendag PC, Schmitz PI, Jansen PP, et al.: Fractionated high-dose-rate brachytherapy in primary carcinoma of the nasopharynx. J Clin Oncol 16 (6): 2213-20, 1998.
- Teo PM, Leung SF, Lee WY, et al.: Intracavitary brachytherapy significantly enhances local control of early T-stage nasopharyngeal carcinoma: the existence of a dose-tumor-control relationship above conventional tumoricidal dose. Int J Radiat Oncol Biol Phys 46 (2): 445-58, 2000.
- Le QT, Tate D, Koong A, et al.: Improved local control with stereotactic radiosurgical boost in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 56 (4): 1046-54, 2003.
Tratamiento del carcinoma de nasofaringe en estadios II, III y IV no metastásico
Opciones de tratamiento del carcinoma de nasofaringe en estadios II, III y IV no metastásico
Las opciones de tratamiento para el carcinoma de nasofaringe en estadios II, III y IV no metastásico son las siguientes:
- Radioterapia.
- Quimiorradioterapia simultánea.
- Quimioterapia neoadyuvante y quimiorradioterapia simultánea.
- Quimiorradioterapia simultánea y quimioterapia adyuvante.
- Quimioterapia neoadyuvante seguida de radioterapia sola. Esta opción está en estudio.
Radioterapia
La radioterapia de dosis alta con quimioterapia es el tratamiento inicial para el carcinoma de nasofaringe. Se administra radioterapia de dosis alta en el sitio del tumor primario y radioterapia profiláctica en los ganglios linfáticos regionales bilaterales del cuello. En algunos estudios se notificó el tratamiento mediante radioterapia de fraccionamiento modificado. La dosis de radioterapia y los márgenes del campo se ajustan de manera individual según la ubicación y el tamaño del tumor primario y los ganglios linfáticos.
La mayoría de los tumores se tratan solo con radioterapia de haz externo (RTE). En algunos pacientes, a veces se refuerza la radioterapia con implantes intracavitarios o intersticiales, o mediante el uso de radiocirugía estereotáctica cuando se cuenta con la pericia clínica y las características anatómicas son las adecuadas.
Evidencia (radioterapia):
- En un ensayo multicéntrico, de ausencia de inferioridad y fase III
(NCT02633202) se evaluó el uso de radioterapia sola versus quimiorradioterapia para pacientes con carcinoma de nasofaringe de riesgo bajo en estadio II, T3, N0, M0 (7ª edición de la estadificación de la American Joint Committee on Cancer). El estudio se hizo en la China endémica, donde la causa de casi todos los casos de carcinoma de nasofaringe es el virus de Epstein-Barr (VEB). En este ensayo, 341 pacientes se asignaron al azar a recibir radioterapia de intensidad modulada (RTIM) sola (n = 172) o quimiorradioterapia simultánea (RTIM con cisplatino 100 mg/m2 cada 3 semanas durante 3 ciclos [n = 169]). El criterio principal de valoración fue la supervivencia sin fracaso (SSF) a 3 años.[Nivel de evidencia B1]
- La tasa de SSF a 3 años fue del 90,5 % en el grupo de RTIM sola y del 91,9 % en el grupo de quimiorradioterapia simultánea (diferencia, −1,4 %; intervalo de confianza [IC] 95 % unilateral, −7,4 % hasta el infinito; P para la ausencia de inferioridad < 0,001). No hubo diferencias en las tasas de supervivencia general (SG), recaída locorregional o metástasis a distancia entre los 2 grupos.
- Los pacientes del grupo de RTIM sola presentaron significativamente menos efectos tóxicos de grados 3 y 4, como toxicidad hematológica y no hematológica (náuseas, vómitos, anorexia, pérdida de peso, mucositis). El grupo de RTIM sola tuvo mejores puntajes de calidad de vida durante la radioterapia.
- Además de que el ensayo se realizó en un área en el que casi todos los casos de carcinoma de nasofaringe se debieron al VEB, todos los pacientes presentaban un valor de corte para el DNA del VEB inferior a 4000 copias/ml para poder participar en el estudio. Asimismo, los pacientes con tumores en estadio II (T1–2, N1) y enfermedad ganglionar debían presentar un tamaño ganglionar menor a 3 cm, sin extensión extraganglionar para poder participar en el estudio.
En este ensayo se demuestra que la radioterapia sola podría usarse para el tratamiento de la enfermedad en estadio limitado si la concentración del VEB (que no suele medirse en los Estados Unidos) es menor de 4000 copias/ml. La radioterapia sola antes no se consideraba tratamiento estándar, pero según estos resultados, es posible sopesar esta posibilidad para los pacientes con menor volumen de enfermedad y concentración vírica baja del VEB.
Quimiorradioterapia
Se han notificado estudios y metanálisis en los que se investigan combinaciones de quimiorradiación.[Nivel de evidencia C1]; En general, en los resultados se notifica un aumento en la supervivencia cuando se añade quimioterapia a la radioterapia.
Evidencia (quimioterapia neoadyuvante vs. quimiorradioterapia):
Los datos de ensayos aleatorizados de fase III avalan la administración de quimioterapia de inducción con gemcitabina y cisplatino antes de la quimiorradioterapia simultánea.
- En un ensayo multicéntrico, aleatorizado, controlado, de fase III (NCT01872962) se comparó la quimioterapia de inducción con gemcitabina y cisplatino y quimiorradioterapia simultánea, con la quimiorradioterapia simultánea sola. Al cabo de una mediana de seguimiento de 42,7 meses, la tasa de supervivencia sin recidiva a 3 años fue del 85,3 % en los pacientes del grupo de quimioterapia de inducción y del 76,5 % en los pacientes del grupo de tratamiento estándar (cociente de riesgo instantáneo estratificado [HR] de recidiva o muerte, 0,51; IC 95 %, 0,34–0,77; P = 0,001).[Nivel de evidencia B1]
En un ensayo multicéntrico de fase III, los pacientes se asignaron al azar a recibir quimiorradioterapia simultánea sola (tratamiento estándar, n = 238) o quimioterapia de inducción con gemcitabina y cisplatino antes de la quimiorradioterapia simultánea (n = 242). Al cabo de una mediana de seguimiento de 69,8 meses, los pacientes en el grupo de quimioterapia de inducción tuvieron una tasa de SG a 5 años más alta (87,9 %) que los del grupo de tratamiento estándar (78,8 %) (CRI, 0,51; IC 95 %, 0,34–0,78; P = 0,001). El riesgo de efectos tóxicos tardíos fue similar (efectos tóxicos de grado 3 o superior, 11,3 vs. 11,4 %).[Nivel de evidencia B1]
Evidencia (quimiorradioterapia y quimioterapia adyuvante):
- La quimiorradioterapia seguida de quimioterapia adyuvante se usó en el ensayo INT-0099.[Nivel de evidencia C1]
- Los pacientes con diseminación parafaríngea en un principio se estadificaron como T3 en el estudio INT-0099 y ahora se consideran T2 según la estadificación vigente.
- La tasa de control a 3 años fue del 91,7 % en el grupo de radioterapia (mediana del periodo de seguimiento, 34 meses) y del 100 % en el grupo de quimiorradioterapia y quimioterapia adyuvante (mediana del periodo de seguimiento, 44 meses) (P =0,10). La tasa de supervivencia sin enfermedad (SSE) a 3 años fue del 91,7 % en el grupo de radioterapia y del 96,9 % en el grupo de quimiorradioterapia y quimioterapia adyuvante (P = 0,66).
Evidencia (quimioterapia combinada y radioterapia vs. radioterapia sola):
- En tres ensayos aleatorizados prospectivos se comparó la quimioterapia combinada (es decir, cisplatino, epirrubicina y bleomicina o cisplatino con infusión de fluorouracilo [5-FU]) y radioterapia, con la radioterapia sola.[Nivel de evidencia A1]; [Nivel de evidencia B1]
- ] Aunque la SSE mejoró en el grupo de quimioterapia, solo en el ensayo Intergroup, en el que se administró quimioterapia de cisplatino junto con radioterapia, se informó de una mejora de la SG en ambos grupos.
Evidencia (quimiorradioterapia con carboplatino vs cisplatino):
- En un estudio con 1355 pacientes se comparó la radioterapia simultánea con carboplatino o cisplatino administrado con una infusión mensual de 96 horas de 5-FU durante 3 ciclos.[Nivel de evidencia A1]
- La tasa de SSE a 3 años fue del 63,4 % en los pacientes del grupo de cisplatino y del 60,9 % en los pacientes del grupo de carboplatino (CRI, 0,70; IC 95 %, 0,50–0,98; P = 0,961).
- Las tasas de SG fueron del 77 % en los pacientes del grupo de cisplatino y del 79 % en los pacientes del grupo de carboplatino (CRI, 0,83; IC 95 %, 0,63–1,010; P = 0,988).
- El grado de toxicidad renal y el recuento de glóbulos rojos fue mayor en los pacientes del grupo de cisplatino.
Cirugía
En ocasiones se indica la disección del cuello en los pacientes con ganglios linfáticos persistentes o recidivantes si se controla el sitio del tumor primario.
Quimioterapia
Se administra quimioterapia a los pacientes con enfermedad en estadio lVC.
Se debe considerar la participación en ensayos clínicos para pacientes con tumores avanzados a fin de evaluar el uso de la quimioterapia antes de la radioterapia, de manera simultánea con la radioterapia, o como terapia adyuvante luego de la radioterapia.
Ensayos clínicos en curso
Realizar una búsqueda avanzada en inglés de los ensayos clínicos sobre cáncer auspiciados por el NCI que ahora aceptan pacientes. La búsqueda se puede simplificar por ubicación del ensayo, tipo de tratamiento, nombre del fármaco y otros criterios. También se dispone de información general sobre los ensayos clínicos.
References
- Wang Q, Xu G, Xia Y, et al.: Comparison of induction chemotherapy plus concurrent chemoradiotherapy and induction chemotherapy plus radiotherapy in locally advanced nasopharyngeal carcinoma. Oral Oncol 111: 104925, 2020.
- Baujat B, Audry H, Bourhis J, et al.: Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 64 (1): 47-56, 2006.
- Xiao WW, Han F, Lu TX, et al.: Treatment outcomes after radiotherapy alone for patients with early-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 74 (4): 1070-6, 2009.
- Johnson CR, Schmidt-Ullrich RK, Wazer DE: Concomitant boost technique using accelerated superfractionated radiation therapy for advanced squamous cell carcinoma of the head and neck. Cancer 69 (11): 2749-54, 1992.
- Chen CY, Han F, Zhao C, et al.: Treatment results and late complications of 556 patients with locally advanced nasopharyngeal carcinoma treated with radiotherapy alone. Br J Radiol 82 (978): 452-8, 2009.
- Perez CA, Devineni VR, Marcial-Vega V, et al.: Carcinoma of the nasopharynx: factors affecting prognosis. Int J Radiat Oncol Biol Phys 23 (2): 271-80, 1992.
- Lee AW, Law SC, Foo W, et al.: Nasopharyngeal carcinoma: local control by megavoltage irradiation. Br J Radiol 66 (786): 528-36, 1993.
- Geara FB, Sanguineti G, Tucker SL, et al.: Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of distant metastasis and survival. Radiother Oncol 43 (1): 53-61, 1997.
- Sanguineti G, Geara FB, Garden AS, et al.: Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of local and regional control. Int J Radiat Oncol Biol Phys 37 (5): 985-96, 1997.
- Mendenhall WM, Werning JW, Pfister DG: Treatment of head and neck cancer. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 729-80.
- Itami J, Anzai Y, Nemoto K, et al.: Prognostic factors for local control in nasopharyngeal cancer (NPC): analysis by multivariate proportional hazard models. Radiother Oncol 21 (4): 233-9, 1991.
- Levendag PC, Schmitz PI, Jansen PP, et al.: Fractionated high-dose-rate brachytherapy in primary carcinoma of the nasopharynx. J Clin Oncol 16 (6): 2213-20, 1998.
- Teo PM, Leung SF, Lee WY, et al.: Intracavitary brachytherapy significantly enhances local control of early T-stage nasopharyngeal carcinoma: the existence of a dose-tumor-control relationship above conventional tumoricidal dose. Int J Radiat Oncol Biol Phys 46 (2): 445-58, 2000.
- Le QT, Tate D, Koong A, et al.: Improved local control with stereotactic radiosurgical boost in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 56 (4): 1046-54, 2003.
- Tang LL, Guo R, Zhang N, et al.: Effect of Radiotherapy Alone vs Radiotherapy With Concurrent Chemoradiotherapy on Survival Without Disease Relapse in Patients With Low-risk Nasopharyngeal Carcinoma: A Randomized Clinical Trial. JAMA 328 (8): 728-736, 2022.
- Cheng SH, Tsai SY, Yen KL, et al.: Concomitant radiotherapy and chemotherapy for early-stage nasopharyngeal carcinoma. J Clin Oncol 18 (10): 2040-5, 2000.
- Al-Sarraf M, LeBlanc M, Giri PG, et al.: Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 16 (4): 1310-7, 1998.
- Teo PM, Chan AT, Lee WY, et al.: Enhancement of local control in locally advanced node-positive nasopharyngeal carcinoma by adjunctive chemotherapy. Int J Radiat Oncol Biol Phys 43 (2): 261-71, 1999.
- Chan AT, Teo PM, Ngan RK, et al.: Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol 20 (8): 2038-44, 2002.
- Huncharek M, Kupelnick B: Combined chemoradiation versus radiation therapy alone in locally advanced nasopharyngeal carcinoma: results of a meta-analysis of 1,528 patients from six randomized trials. Am J Clin Oncol 25 (3): 219-23, 2002.
- Lin JC, Jan JS, Hsu CY, et al.: Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 21 (4): 631-7, 2003.
- Chua DT, Ma J, Sham JS, et al.: Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol 23 (6): 1118-24, 2005.
- Wee J, Tan EH, Tai BC, et al.: Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 23 (27): 6730-8, 2005.
- Zhang L, Zhao C, Peng PJ, et al.: Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. J Clin Oncol 23 (33): 8461-8, 2005.
- Baujat B, Audry H, Bourhis J, et al.: Chemotherapy as an adjunct to radiotherapy in locally advanced nasopharyngeal carcinoma. Cochrane Database Syst Rev (4): CD004329, 2006.
- Chen Y, Liu MZ, Liang SB, et al.: Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of china. Int J Radiat Oncol Biol Phys 71 (5): 1356-64, 2008.
- Lee AW, Tung SY, Chua DT, et al.: Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 102 (15): 1188-98, 2010.
- Lee AW, Tung SY, Chan AT, et al.: A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiother Oncol 98 (1): 15-22, 2011.
- Lee AW, Tung SY, Ngan RK, et al.: Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer 47 (5): 656-66, 2011.
- Zhang Y, Chen L, Hu GQ, et al.: Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med 381 (12): 1124-1135, 2019.
- Blanchard P, Lee AWM, Carmel A, et al.: Meta-analysis of chemotherapy in nasopharynx carcinoma (MAC-NPC): An update on 26 trials and 7080 patients. Clin Transl Radiat Oncol 32: 59-68, 2022.
- Zhang Y, Chen L, Hu GQ, et al.: Final Overall Survival Analysis of Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma: A Multicenter, Randomized Phase III Trial. J Clin Oncol 40 (22): 2420-2425, 2022.
- Sun Y, Li WF, Chen NY, et al.: Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17 (11): 1509-1520, 2016.
- Yang Q, Cao SM, Guo L, et al.: Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer 119: 87-96, 2019.
- Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV(> or = N2, M0) undifferentiated nasopharyngeal carcinoma: a positive effect on progression-free survival. International Nasopharynx Cancer Study Group. VUMCA I trial. Int J Radiat Oncol Biol Phys 35 (3): 463-9, 1996.
- Lee AW, Lau WH, Tung SY, et al.: Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 23 (28): 6966-75, 2005.
- Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, et al.: Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur J Cancer 43 (9): 1399-406, 2007.
- Ma BB, Tannock IF, Pond GR, et al.: Chemotherapy with gemcitabine-containing regimens for locally recurrent or metastatic nasopharyngeal carcinoma. Cancer 95 (12): 2516-23, 2002.
- Dimery IW, Peters LJ, Goepfert H, et al.: Effectiveness of combined induction chemotherapy and radiotherapy in advanced nasopharyngeal carcinoma. J Clin Oncol 11 (10): 1919-28, 1993.
- Chan AT, Teo PM, Leung TW, et al.: A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 33 (3): 569-77, 1995.
- Merlano M, Benasso M, Corvò R, et al.: Five-year update of a randomized trial of alternating radiotherapy and chemotherapy compared with radiotherapy alone in treatment of unresectable squamous cell carcinoma of the head and neck. J Natl Cancer Inst 88 (9): 583-9, 1996.
- Jeremic B, Shibamoto Y, Milicic B, et al.: Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol 18 (7): 1458-64, 2000.
Tratamiento del carcinoma de nasofaringe metastásico y recidivante
Opciones de tratamiento del carcinoma de nasofaringe metastásico y recidivante
Las opciones de tratamiento para el carcinoma de nasofaringe metastásico y recidivante son las siguientes:
- Radioterapia.
Radioterapia
El tratamiento inicial para los pacientes con carcinoma de nasofaringe son dosis altas de radioterapia dirigida al sitio del tumor primario y el cuello con quimioterapia. Determinados pacientes con recidiva local se pueden tratar de nuevo con dosis moderadas de radioterapia de haz externo si se usa radioterapia de intensidad modulada, radioterapia estereotáctica o radiación intracavitaria o intersticial dirigida al sitio de la recidiva.; [Nivel de evidencia C2]; [Nivel de evidencia C3] La dosis de radioterapia y los márgenes del campo se ajustan de manera individual según la ubicación y el tamaño del tumor primario y los ganglios linfáticos.
La mayoría de los tumores se tratan solo con radioterapia de haz externo (RTE). En algunos pacientes, a veces se refuerza la radioterapia con implantes intracavitarios o intersticiales, o mediante el uso de radiocirugía estereotáctica cuando se cuenta con la pericia clínica y las características anatómicas son las adecuadas.
Cirugía
En pacientes muy específicos, se puede considerar la resección quirúrgica de lesiones locales recidivantes.
Quimioterapia
Si un paciente tiene enfermedad metastásica o recidiva local que ya no es susceptible de cirugía o radioterapia, se deberá considerar el uso de quimioterapia.
Evidencia (quimioterapia):
- En un ensayo de fase III multicéntrico, aleatorizado, sin enmascaramiento se incluyeron pacientes con carcinoma de nasofaringe metastásico o recidivante de 22 hospitales de China. Los pacientes se asignaron al azar en una proporción 1:1 para recibir gemcitabina (1 g/m2 intravenosa [IV] los días 1 y 8) y cisplatino (80 mg/m2 IV el día 1), o fluorouracilo ([5-FU] 4 g/m2 en infusión IV continua durante 96 horas) y cisplatino (80 mg/m2 IV el día 1) 1 vez cada 3 semanas durante un máximo de 6 ciclos.[Nivel de evidencia B1] De los 362 pacientes, 181 se asignaron al grupo de gemcitabina con cisplatino y 181 al grupo de 5-FU con cisplatino.
- La mediana de seguimiento para la supervivencia sin progresión (SSP) fue de 19,4 meses (intervalo intercuartílico [IIC], 12,1–35,6). La mediana de SSP fue de 7,0 meses (intervalo, 4,4–10,9) en el grupo de gemcitabina y de 5,6 meses (intervalo 3,0–7,0) en el grupo de 5-FU (cociente de riesgos instantáneos 0,55; intervalo de confianza del 95 %, 0,44–0,68; P< 0,0001).
- Hubo diferencias significativas en las incidencias de los siguientes eventos adversos relacionados con el tratamiento de grado 3 o 4:
- Leucopenia (52 [29 %] en el grupo de gemcitabina vs. 15 [9 %] en el grupo de 5-FU; P< 0,0001).
- Neutropenia (41 [23 %] en el grupo de gemcitabina vs. 23 [13 %] en el grupo de 5-FU; P = 0,0251).
- Trombocitopenia (24 [13 %] en el grupo de gemcitabina vs. 3 [2 %] en el grupo de 5-FU; P = 0,0007).
- Inflamación de la mucosa (0 en el grupo de gemcitabina vs. 25 [14 %] en el grupo de 5-FU; P< 0,0001).
- Se presentaron eventos adversos graves relacionados con el tratamiento en 7 pacientes (4 %) del grupo de gemcitabina y en 10 pacientes (6 %) del grupo de 5-FU.
- Seis pacientes (3 %) del grupo de gemcitabina y 14 pacientes (8 %) del grupo de 5-FU interrumpieron el tratamiento debido a eventos adversos relacionados con el medicamento.
- En ninguno de los dos grupos hubo muertes relacionadas con el tratamiento.
Ensayos clínicos en curso
Realizar una búsqueda avanzada en inglés de los ensayos clínicos sobre cáncer auspiciados por el NCI que ahora aceptan pacientes. La búsqueda se puede simplificar por ubicación del ensayo, tipo de tratamiento, nombre del fármaco y otros criterios. También se dispone de información general sobre los ensayos clínicos.
References
- Baujat B, Audry H, Bourhis J, et al.: Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 64 (1): 47-56, 2006.
- Mendenhall WM, Werning JW, Pfister DG: Treatment of head and neck cancer. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 729-80.
- Vikram B, Strong EW, Shah JP, et al.: Intraoperative radiotherapy in patients with recurrent head and neck cancer. Am J Surg 150 (4): 485-7, 1985.
- Koutcher L, Lee N, Zelefsky M, et al.: Reirradiation of locally recurrent nasopharynx cancer with external beam radiotherapy with or without brachytherapy. Int J Radiat Oncol Biol Phys 76 (1): 130-7, 2010.
- Lu JJ, Shakespeare TP, Tan LK, et al.: Adjuvant fractionated high-dose-rate intracavitary brachytherapy after external beam radiotherapy in Tl and T2 nasopharyngeal carcinoma. Head Neck 26 (5): 389-95, 2004.
- Tate DJ, Adler JR, Chang SD, et al.: Stereotactic radiosurgical boost following radiotherapy in primary nasopharyngeal carcinoma: impact on local control. Int J Radiat Oncol Biol Phys 45 (4): 915-21, 1999.
- Chua DT, Sham JS, Kwong PW, et al.: Linear accelerator-based stereotactic radiosurgery for limited, locally persistent, and recurrent nasopharyngeal carcinoma: efficacy and complications. Int J Radiat Oncol Biol Phys 56 (1): 177-83, 2003.
- Pai PC, Chuang CC, Wei KC, et al.: Stereotactic radiosurgery for locally recurrent nasopharyngeal carcinoma. Head Neck 24 (8): 748-53, 2002.
- Xiao J, Xu G, Miao Y: Fractionated stereotactic radiosurgery for 50 patients with recurrent or residual nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 51 (1): 164-70, 2001.
- Perez CA, Devineni VR, Marcial-Vega V, et al.: Carcinoma of the nasopharynx: factors affecting prognosis. Int J Radiat Oncol Biol Phys 23 (2): 271-80, 1992.
- Lee AW, Law SC, Foo W, et al.: Nasopharyngeal carcinoma: local control by megavoltage irradiation. Br J Radiol 66 (786): 528-36, 1993.
- Geara FB, Sanguineti G, Tucker SL, et al.: Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of distant metastasis and survival. Radiother Oncol 43 (1): 53-61, 1997.
- Sanguineti G, Geara FB, Garden AS, et al.: Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of local and regional control. Int J Radiat Oncol Biol Phys 37 (5): 985-96, 1997.
- Itami J, Anzai Y, Nemoto K, et al.: Prognostic factors for local control in nasopharyngeal cancer (NPC): analysis by multivariate proportional hazard models. Radiother Oncol 21 (4): 233-9, 1991.
- Levendag PC, Schmitz PI, Jansen PP, et al.: Fractionated high-dose-rate brachytherapy in primary carcinoma of the nasopharynx. J Clin Oncol 16 (6): 2213-20, 1998.
- Teo PM, Leung SF, Lee WY, et al.: Intracavitary brachytherapy significantly enhances local control of early T-stage nasopharyngeal carcinoma: the existence of a dose-tumor-control relationship above conventional tumoricidal dose. Int J Radiat Oncol Biol Phys 46 (2): 445-58, 2000.
- Le QT, Tate D, Koong A, et al.: Improved local control with stereotactic radiosurgical boost in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 56 (4): 1046-54, 2003.
- Al-Sarraf M: Head and neck cancer: chemotherapy concepts. Semin Oncol 15 (1): 70-85, 1988.
- Jacobs C, Lyman G, Velez-García E, et al.: A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol 10 (2): 257-63, 1992.
- Foo KF, Tan EH, Leong SS, et al.: Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol 13 (1): 150-6, 2002.
- Zhang L, Huang Y, Hong S, et al.: Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 388 (10054): 1883-1892, 2016.
Actualizaciones más recientes a este resumen (04/05/2024)
Los resúmenes del PDQ con información sobre el cáncer se revisan con regularidad y se actualizan a medida que se obtiene nueva información. Esta sección describe los cambios más recientes hechos introducidos en este resumen a partir de la fecha arriba indicada.
Aspectos generales de las opciones de tratamiento del carcinoma de nasofaringe
Se añadió Administración de fluorouracilo como subsección nueva.
Tratamiento del carcinoma de nasofaringe en estadios II, III y IV no metastásico
La subsección Radioterapia fue objeto de revisión integral.
Se añadió a Blanchard et al. como referencia 31.
Se añadió texto para indicar que los datos de ensayos aleatorizados de fase III avalan la administración de quimioterapia de inducción con gemcitabina y cisplatino antes de la quimiorradioterapia simultánea (se citó a Zhang et al., Sun et al. y Yang et al. como referencias 32, 33 y 34, respectivamente).
Se añadió texto sobre 2 ensayos aleatorizados de fase III en los que se comparó la quimioterapia de inducción con gemcitabina y cisplatino y quimiorradioterapia simultánea, con la quimiorradioterapia simultánea sola (se añadió un nivel de evidencia B1).
El Consejo editorial del PDQ sobre el tratamiento para adultos es responsable de la redacción y actualización de este resumen y mantiene independencia editorial respecto del NCI. El resumen refleja una revisión independiente de la bibliografía médica y no representa las políticas del NCI ni de los NIH. Para obtener más información sobre las políticas relativas a los resúmenes y la función de los consejos editoriales del PDQ responsables de su actualización, consultar Información sobre este resumen del PDQ e Información del PDQ® sobre el cáncer dirigida a profesionales de la salud.
Información sobre este resumen del PDQ
Propósito de este resumen
Este resumen de información del PDQ sobre el cáncer dirigido a profesionales de la salud proporciona información integral revisada por expertos y basada en la evidencia sobre the treatment of adult nasopharyngeal carcinoma. El objetivo es servir como fuente de información y ayuda para los profesionales clínicos durante la atención de pacientes. No ofrece pautas ni recomendaciones formales para tomar decisiones relacionadas con la atención sanitaria.
Revisores y actualizaciones
El Consejo editorial del PDQ sobre el tratamiento para adultos, que mantiene independencia editorial respecto del Instituto Nacional del Cáncer (NCI), revisa este resumen de manera periódica y, en caso necesario, lo actualiza. Este resumen es el resultado de una revisión bibliográfica independiente y no constituye una declaración de política del NCI ni de los Institutos Nacionales de la Salud (NIH).
Cada mes, los integrantes de este consejo revisan los artículos publicados recientemente para determinar lo siguiente:
- Si el artículo se debe analizar en una reunión del consejo.
- Si conviene añadir texto acerca del artículo.
- Si se debe reemplazar o actualizar un artículo que ya se citó.
Los cambios en los resúmenes se deciden mediante consenso de los integrantes del consejo después de evaluar la solidez de la evidencia de los artículos publicados y determinar la forma de incorporar el artículo en el resumen.
Los revisores principales del sumario sobre Tratamiento del carcinoma de nasofaringe son:
- Andrea Bonetti, MD (Azienda ULSS 9 of the Veneto Region)
- Monaliben Patel, MD (Advocate Christ Medical Center)
- Minh Tam Truong, MD (Boston University Medical Center)
Cualquier comentario o pregunta sobre el contenido de este resumen se debe enviar al Servicio de Información de Cáncer del Instituto Nacional del Cáncer. Por favor, no enviar preguntas o comentarios directamente a los integrantes del consejo, ya que no responderán consultas de manera individual.
Niveles de evidencia
Algunas de las referencias bibliográficas de este resumen se acompañan del nivel de evidencia. El propósito de esto es ayudar al lector a evaluar la solidez de la evidencia que respalda el uso de ciertas intervenciones o abordajes. El Consejo editorial del PDQ sobre el tratamiento para adultos emplea un sistema de jerarquización formal para asignar los niveles de evidencia científica.
Permisos para el uso de este resumen
PDQ (Physician Data Query) es una marca registrada. Se autoriza el uso del texto de los documentos del PDQ; sin embargo, no se podrá identificar como un resumen de información sobre cáncer del PDQ del NCI, salvo que el resumen se reproduzca en su totalidad y se actualice de manera periódica. Por otra parte, se permitirá que un autor escriba una oración como “En el resumen del PDQ del NCI de información sobre la prevención del cáncer de mama se describen, de manera concisa, los siguientes riesgos: [incluir fragmento del resumen]”.
Se sugiere citar la referencia bibliográfica de este resumen del PDQ de la siguiente forma:
PDQ® sobre el tratamiento para adultos. PDQ Tratamiento del carcinoma de nasofaringe. Bethesda, MD: National Cancer Institute. Actualización:
Las imágenes en este resumen se reproducen con autorización del autor, el artista o la editorial para uso exclusivo en los resúmenes del PDQ. La utilización de las imágenes fuera del PDQ requiere la autorización del propietario, que el Instituto Nacional del Cáncer no puede otorgar. Para obtener más información sobre el uso de las ilustraciones de este resumen o de otras imágenes relacionadas con el cáncer, consultar Visuals Online, una colección de más de 2000 imágenes científicas.
Cláusula sobre el descargo de responsabilidad
Según la solidez de la evidencia, las opciones de tratamiento se clasifican como “estándar” o “en evaluación clínica”. Estas clasificaciones no se deben utilizar para justificar decisiones sobre reembolsos de seguros. Para obtener más información sobre la cobertura de seguros, consultar la página Manejo de la atención del cáncer en Cancer.gov/espanol.
Comuníquese con el Instituto Nacional del Cáncer
Para obtener más información sobre las opciones para comunicarse con el NCI, incluso la dirección de correo electrónico, el número telefónico o el chat, consultar la página del Servicio de Información de Cáncer del Instituto Nacional del Cáncer.