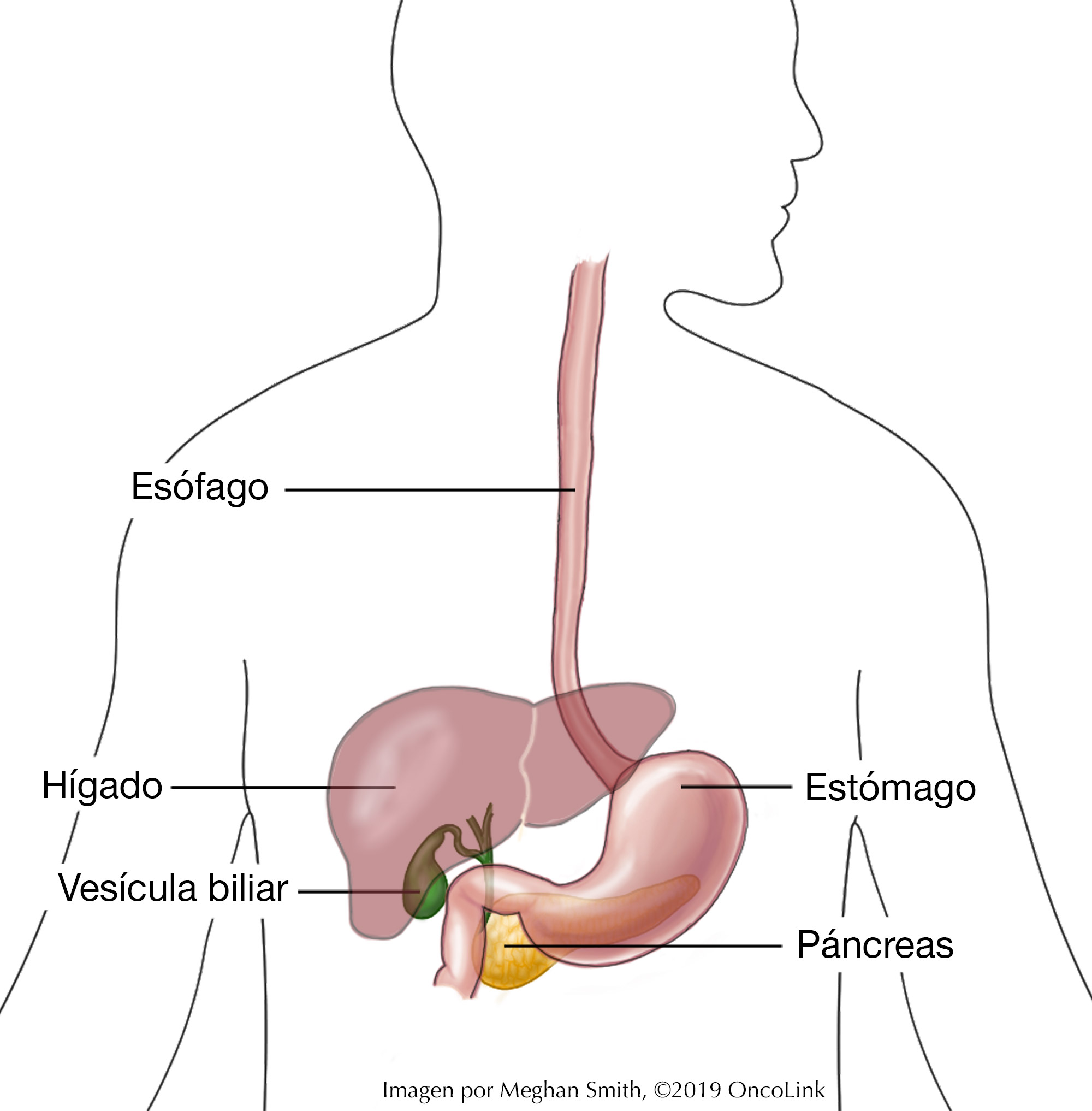
Esophageal Cancer: Staging and Treatment
What is staging for cancer?
Staging is the process of learning how much cancer is in your body and where it is. For esophageal cancer, imaging tests like a barium swallow, endoscopy, endoscopic ultrasound, CT, MRI, and/or PET scans may be used to help stage your cancer. Your providers need to know about your cancer and your health so that they can plan the best treatment for you.
Staging looks at the size of the tumor and where it is, and if it has spread to other organs. The most common staging system used for esophageal cancer is called the “TNM system,” as described by the American Joint Committee on Cancer. It may also be called a pathological stage. Part of this staging is determined by looking at tissue removed during surgery along with other imaging tests. It has three parts:
- T-describes the size/location/extent of the "primary" tumor in the esophagus and how far it has grown into the wall of the esophagus.
- N-describes if the cancer has spread to the lymph nodes.
- M-describes if the cancer has spread to other organs (metastases).
Staging for esophageal cancer also includes “G” which is the histologic stage (how the cells appear under a microscope) or grade. The T, N, M, and G are combined to come up with a stage from 0-IV (0 to 4), with IV being the most advanced.
Depending on how your cancer is treated, other staging systems may be used. If surgery is not done or won’t be done until after other treatment is given, you will be given a clinical stage. The clinical stage is based on a physical exam and imaging tests. If treatments such as chemotherapy or radiation are done before surgery, you will be given a stage, called a post-neoadjuvant stage, after surgery is done.
How is esophageal cancer staged?
Staging for esophageal cancer is based on:
- The location and size of the tumor.
- If the tumor has spread to the lymph nodes. If so, how many lymph nodes are affected.
- If the tumor has spread to other organs. This is called metastasis.
- Whether the cancer is squamous cell type or an adenocarcinoma (location does not affect stage).
Staging is important because it helps guide your treatment options. Below is a summary of the pathological staging system. It is the most used staging system for esophageal cancer. There is also one system for squamous cell carcinoma and one for adenocarcinoma. Talk to your provider about the stage of your cancer.
Squamous Cell Carcinoma
Stage 0: The cancer is in the epithelium only and has not spread. This stage is also called high-grade dysplasia. It is not given a cancer grade.
Stage IA: Cancer is in the lamina propria or muscularis mucosa and has not spread. It is grade I or an unknown grade.
Stage IB: The cancer is in the lamina propria, muscularis mucosa, submucosa or thick muscle layer but has not spread to lymph nodes or distant organs. It can be any grade or an unknown grade.
Stage IIA: The cancer is in the muscularis propria but has not spread to lymph nodes or organs. It is an unknown grade, grade 2, or grade 3; OR The cancer is growing into the outer layer of the esophagus and has not spread to nearby lymph nodes or organs. It is any grade in the lower esophagus or Grade 1 and in the upper or middle esophagus.
Stage IIB: The cancer is growing into the outer layer of the esophagus and has not spread to lymph nodes or organs. It is grade 2 or 3 and found in the upper or middle esophagus OR an unknown grade anywhere in the esophagus OR any grade with an unknown location; OR The cancer is found in the lamina propria, muscularis mucosa, or the submucosa and has spread to 1 or 2 nearby lymph nodes. It can be any grade anywhere in the esophagus.
Stage IIIA: The cancer is in the lamina propria, muscularis mucosa, submucosa, or thick muscle layer and has spread to 6 lymph nodes or less and not to distant organs. It can be any grade.
Stage IIIB: The cancer is in the muscularis propria and spread to 6 lymph nodes or less OR it has grown into the outer layer of the esophagus and spread to 6 lymph nodes or less OR it has grown into the pleura, the pericardium, or the diaphragm and no more than 2 lymph nodes. It has not spread to any organs and it can be any grade.
Stage IVA: The cancer is in the pleura, the pericardium, or the diaphragm and is in 6 or less lymph nodes OR it has grown into the trachea, aorta, spine or other crucial structure in the body and no more than 6 lymph nodes OR any layer of the esophagus and 7 or more lymph nodes. It has not spread to distant organs and it can be any grade.
Stage IVB: The cancer has spread to distant lymph nodes and/or other organs and the cancer is any grade.
Adenocarcinoma
Stage 0: This stage is also called high-grade dysplasia. The cancer is only in the epithelium and has not grown into deeper layers. It has not spread and cancer grade does not apply.
Stage IA: The cancer is in the lamina propria or the muscularis mucosa and has not spread. It is grade 1 or an unknown grade.
Stage IB: The cancer is in the lamina propria, muscularis mucosa or the submucosa. It has not spread and is grade 1, 2, or unknown.
Grade IC: The cancer is in the lamina propria, muscularis mucosa, submucosa, or the thick muscle layer and has not spread to the lymph nodes or organs. It can be grade 1, 2, or 3.
Stage IIA: The cancer is in the muscularis propria, has not spread to lymph nodes or organs, and can be any grade.
Stage IIB: The cancer is growing into the lamina propria, muscularis mucosa, or submucosa. It has spread to 1 or 2 lymph nodes but not to distant organs and it can be any grade OR the cancer is in the outer layer of the esophagus, has not spread to lymph nodes, and can be any grade.
Stage IIIA: The cancer is in the lamina propria, muscularis mucosa, submucosa, or the thick layer of muscle. It has spread to 6 or less lymph nodes and not to distant organs. It can be any grade.
Stage IIIB: The cancer is growing into the thick muscle layer and is in no more than 6 lymph nodes OR the outer layer of the esophagus and spread to 6 lymph nodes or less OR the pleura, the pericardium, or the diaphragm and to no more than 2 nearby lymph nodes. It has not spread to organs and can be any grade.
Stage IVA: The cancer is growing into the pleura, the pericardium, or the diaphragm and to no more than 6 lymph nodes OR the trachea, the aorta, the spine, or other critical structure and 6 or less lymph nodes OR any layers of the esophagus and 7 or more nearby lymph nodes. It has not spread to distant organs and it can be any grade.
Stage IVB: The cancer has spread to distant lymph nodes and/or other organs, and it can be any grade.
Another aspect of esophageal cancer staging is whether the cancer is resectable or not. This means, can the cancer be removed completely with surgery. Often, stage 0, I, and II are resectable. Most often stage III is resectable even if it has metastasized to the nearby lymph nodes. Stage IV cancers are not resectable. Your provider will talk to you about your options for surgery.
How is esophageal cancer treated?
Treatment for esophageal cancer is based on the size and location of the tumor, if it has spread to the lymph nodes or other organs, and your overall health. There can be more than one type of treatment used to treat esophageal cancer. Some of the treatments used are:
- Surgery.
- Chemotherapy.
- Targeted Therapy.
- Immunotherapy.
- Radiation.
- Photodynamic Therapy.
- Clinical Trials.
Surgery
Surgery is used to treat some cases of esophageal cancer. An esophagectomy, which is the removal of part or all of the esophagus along with lymph nodes, is one type of surgery. In this surgery, the esophagus (sometimes the top part of the esophagus and part of the stomach) is removed. The stomach is then pulled up into your chest so that food can still pass from your mouth into your stomach. It can be done by making large incisions or many small incisions. This is a very complicated surgery and there are potential side effects like bleeding, strictures (narrowing where the esophagus meets the stomach), voice changes, or a leak forming at the connection between the throat and stomach. You need to be very healthy to be able to tolerate this surgery. Talk with your provider about whether this surgery may be part of your treatment plan.
A less extensive procedure called an endoscopic mucosal resection may be done. An endoscope (thin tube with a light and tools attached to the end of it) is passed down through your throat and the tumor is removed. This is used for pre-cancerous or small, early-stage cancers.
Surgery may also be used to treat the side effects of the cancer. This is called palliative surgery and it is not meant to treat the cancer itself, but to help you be more comfortable. Examples of palliative surgery are feeding tube placement and esophageal dilation (a procedure used to stretch the tissues in an area of your esophagus so that you can swallow easier).
Chemotherapy
Chemotherapy is the use of anti-cancer medications to treat cancer. It is often given with radiation, called chemoradiation. It can be used after surgery (adjuvant) or before surgery (neoadjuvant). It can also be used to shrink esophageal cancer cells that have metastasized to other parts of the body. Chemotherapy medications used are carboplatin, paclitaxel, oxaliplatin, 5-Fu, capecitabine, cisplatin, irinotecan, epirubicin, docetaxel, and trifluridine/tipiracil. Side effects of chemotherapy can include nausea and vomiting, fatigue, diarrhea, constipation, mouth sores, hair loss, and loss of appetite. Many of these side effects can be managed with medications or changes in diet. Talk to your provider about what specific side effects you should expect with the chemotherapies you are getting.
Targeted Therapy
Targeted therapies can be used with chemotherapy or by themselves. Targeted therapies target specific changes on a cell that help cancer grow and spread. Different targeted therapies are used depending on the target found on a cancer cell.
- Trastuzumab and Fam-trastuzumab deruxtecan target HER2.
- Ramucirumab blocks VEGF and stops the body from making more blood vessels that can feed the tumor.
- Entrectinib and Larotrectinib are TRK inhibitors and work to stop the growth of cancer cells.
Targeted therapies can also cause side effects like fatigue, nausea, vomiting, and diarrhea. Medications and changes in diet can help manage these side effects.
Immunotherapy
Immunotherapy uses your body’s own immune system to find and kill cancer cells. It is also called biologic therapy. Immunotherapy medications being used to treat esophageal cancer are ipilimumab, nivolumab, and pembrolizumab.
Radiation Therapy
Radiation therapy can be used in different ways to treat esophageal cancer. It may be used along with chemotherapy (called chemoradiation), before surgery to make a tumor smaller and easier to remove (neoadjuvant), after surgery (adjuvant), or as a palliative therapy (therapy meant to manage symptoms of the cancer). The two types of radiation therapy that are used are external-beam radiation therapy and internal radiation therapy (brachytherapy). Side effects of radiation can be skin changes, fatigue, mouth and throat sores, thickened saliva, and changes in your bowels.
Photodynamic Therapy
Photodynamic therapy is the use of a light-activated drug called porfimer sodium that is placed into a vein and collects in cancer cells. A laser light is then used to change the drug into a chemical that kills the cancer cells. The dead cancer cells are removed during an upper endoscopy. It is used to treat pre-cancerous lesions in the esophagus, early-stage cancers, and large tumors that are blocking the esophagus. This procedure can be done more than once but cannot get into cells that are in deeper tissues.
Clinical Trials
You may be offered a clinical trial as part of your treatment plan. To find out more about current clinical trials, visit the OncoLink Clinical Trials Matching Service.
Making Treatment Decisions
Your care team will make sure you are included in choosing your treatment plan. This can be overwhelming as you may be given a few options to choose from. It feels like an emergency, but you can take a few weeks to meet with different providers and think about your options and what is best for you. This is a personal decision. Friends and family can help you talk through the options and the pros and cons of each, but they cannot make the decision for you. You need to be comfortable with your decision – this will help you move on to the next steps. If you ever have any questions or concerns, be sure to call your team.
You can learn more about esophageal cancer at OncoLink.org.
Referencias
American Cancer Society. Esophagus Cancer.
NCCN Guidelines, Esophageal and Esophagogastric Junction Cancers, Version 2.2023. March 10, 2023. (log in required).
Burt, B. M., Groth, S. S., Sada, Y. H., Farjah, F., Cornwell, L., Sugarbaker, D. J., & Massarweh, N. N. (2017). Utility of Adjuvant Chemotherapy After Neoadjuvant Chemoradiation and Esophagectomy for Esophageal Cancer. Annals of Surgery.
D’Journo, X. B., & Thomas, P. A. (2014). Current management of esophageal cancer. Journal of Thoracic Disease, 6(2), S253-S264.
Haefner, M. F., Lang, K., Krug, D., Koerber, S. A., Uhlmann, L., Kieser, M., ... & Sterzing, F. (2015). Prognostic factors, patterns of recurrence, and toxicity for patients with esophageal cancer undergoing definitive radiotherapy or chemo-radiotherapy. Journal of Radiation Research, 56(4), 742-749.
Keith, B. (2016). Esophageal Cancer. Mosby's Oncology Nursing Advisor: A Comprehensive Guide to Clinical Practice, 58.
Kelly, R. J., Ajani, J. A., Kuzdzal, J., Zander, T., Van Cutsem, E., Piessen, G., ... & Moehler, M. (2021). Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. New England Journal of Medicine, 384(13), 1191-1203.
Purim, O., Beny, A., Inbar, M., Shulman, K., Brenner, B., Dudnik, E., ... & Sarid, D. (2018). Biomarker-driven therapy in metastatic gastric and esophageal cancer: Real-life clinical experience. Targeted oncology, 1-10.
Saltzman, J. R., Gibson, M. K., & Goldberg, R. M. (2018). Clinical manifestations, diagnosis, and staging of esophageal cancer. UpToDate, Waltham, MA.
Shaheen, N. J., Falk, G. W., Iyer, P. G., & Gerson, L. B. (2016). ACG clinical guideline: diagnosis and management of Barrett’s esophagus. The American Journal of Gastroenterology, 111(1), 30-50.
Takeuchi, M., Suda, K., Hamamoto, Y., Kato, M., Mayanagi, S., Yoshida, K., ... & Takeuchi, H. (2018). Technical feasibility and oncologic safety of diagnostic endoscopic resection for superficial esophageal cancer. Gastrointestinal endoscopy, 88(3), 456-465.
Talukdar, F. R., di Pietro, M., Secrier, M., Moehler, M., Goepfert, K., Lima, S. S. C., ... & Herceg, Z. (2018). Molecular landscape of esophageal cancer: implications for early detection and personalized therapy. Annals of the New York Academy of Sciences, 1434(1), 342-359.
Watanabe, M., Otake, R., Kozuki, R., Toihata, T., Takahashi, K., Okamura, A., & Imamura, Y. (2020). Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surgery today, 50(1), 12-20.