Instituto Nacional del Cáncer
Fecha de publicación: Feb 16, 2023
Resumen de información revisada por expertos acerca del tratamiento del cáncer de endometrio.
Tratamiento del cáncer de endometrio
Información general sobre el cáncer de endometrio
El cáncer de endometrio es la neoplasia maligna ginecológica más común en los Estados Unidos: representa el 7 % de todos los cánceres en mujeres. La mayoría de los casos se diagnostican en estadio temprano y son susceptibles de tratamiento con cirugía sola. Sin embargo, las pacientes con características patológicas que predicen una tasa alta de recaída y las pacientes con metástasis extrauterinas en el momento del diagnóstico tienen una tasa alta de recaída incluso tras recibir terapia adyuvante.
Incidencia y mortalidad
Número estimado de casos nuevos y defunciones por cáncer en el cuerpo del útero, que incluye el endometrio, en los Estados Unidos para 2023:
- Casos nuevos: 66 200.
- Defunciones: 13 030.
En general, el cáncer de endometrio se diagnostica y trata en estadio temprano. La enfermedad cardiovascular es la causa más común de muerte en pacientes de cáncer de endometrio debido a los factores de riesgo metabólico relacionados.
Características anatómicas
El endometrio es el revestimiento más interno del útero y tiene capas funcionales y basales. La capa funcional es sensible a las hormonas y se desprende de modo cíclico durante la menstruación de las mujeres en edad reproductiva. Tanto el estrógeno como la progesterona son necesarios para mantener el revestimiento endometrial en condiciones normales. Sin embargo, los factores que conducen a un exceso de estrógeno, como la obesidad y la anovulación, aumentan los depósitos del revestimiento endometrial. Estos cambios pueden causar hiperplasia en el endometrio y, en algunos casos, cáncer de endometrio. Cualquiera que sea su causa, el engrosamiento del revestimiento conducirá a un desprendimiento del tejido endometrial a través del conducto endocervical hacia la vagina. Como resultado, el sangrado menstrual abundante o el sangrado posmenopáusico son, con frecuencia, los signos iniciales del cáncer de endometrio. Como este síntoma tiende a presentarse temprano en el curso de la enfermedad, permite identificar la enfermedad en un estadio temprano en la mayoría de las mujeres.
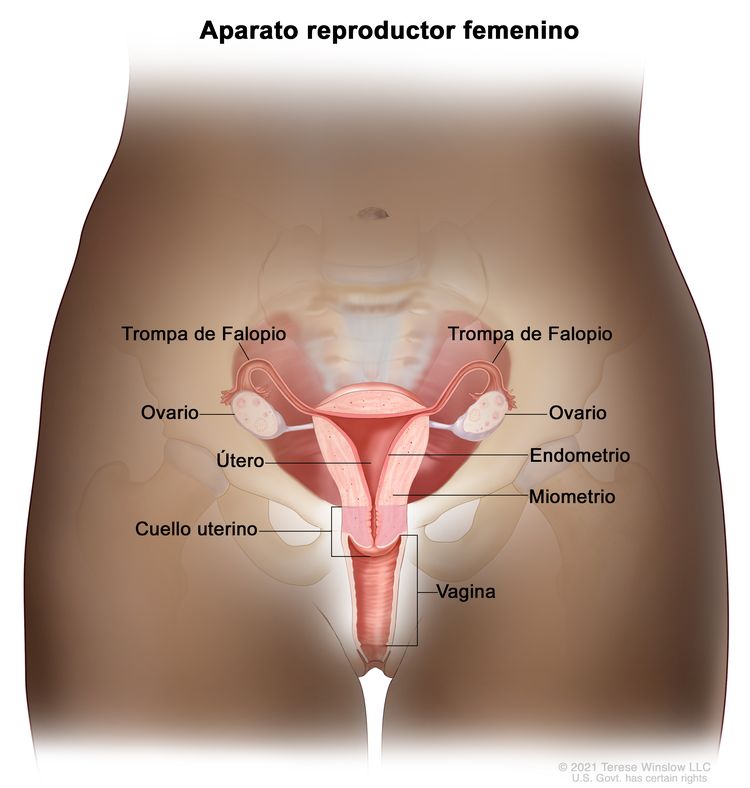 Anatomía del aparato reproductor femenino
Anatomía del aparato reproductor femenino
Factores de riesgo
endometrial cancerEl envejecimiento es el factor de riesgo más importante para la mayoría de cánceres. Otros factores de riesgo del cáncer de endometrio son los siguientes:
- Terapia hormonal.
- Terapia con estrógenos en la posmenopausia.
- Modificadores selectivos de los receptores de estrógeno.
- Terapia con tamoxifeno.
- Obesidad.
- Síndrome metabólico.
- Diabetes.
- Factores reproductivos.
- Nuliparidad.
- Menarquia temprana o menopausia tardía.
- Síndrome de ovario poliquístico.
- Antecedentes familiares o predisposición genética.
- Madre, hermana o hija con cáncer de útero.
- Ciertos síndromes genéticos, como el síndrome de Lynch.
- Hiperplasia del endometrio.
Para obtener más información, consultar Prevención del cáncer de endometrio.
La exposición prolongada y sin oposición a estrógenos se ha relacionado con un aumento de riesgo de cáncer de endometrio. Sin embargo la terapia combinada de estrógeno y progesterona previene el aumento de este riesgo.
El tamoxifeno, que se usa para prevenir y tratar el cáncer de mama NSABP-B-14, se relaciona con un aumento de riesgo de cáncer de endometrio relacionado con el efecto estrogénico del tamoxifeno en el endometrio. Es importante que las pacientes que reciben tamoxifeno y presentan un sangrado uterino anómalo se sometan a exámenes de seguimiento y biopsia del revestimiento endometrial. La Administración de Alimentos y Medicamentos de los Estados Unidos (FDA) emitió una advertencia en recuadro negro que incluye datos sobre el aumento de neoplasias uterinas malignas relacionadas con el uso del tamoxifeno.
Características clínicas
El sangrado vaginal irregular es el signo de presentación más común del cáncer de endometrio. En general, ocurre al comienzo de la enfermedad y es la razón por la que a la mayoría de las pacientes se les diagnostica cáncer de endometrio en estadio I, que es muy curable.
Evaluación diagnóstica
Para diagnosticar un cáncer de cuello uterino, es posible utilizar los siguientes procedimientos:
- Ecografía transvaginal.
- Biopsia del endometrio.
- Examen pélvico.
- Dilatación y legrado (DyL).
- Histeroscopia.
Para el diagnóstico definitivo del cáncer de endometrio se necesita realizar un procedimiento para extraer muestras directamente del tejido endometrial.
El frotis de Pap no es un procedimiento confiable para detectar el cáncer de endometrio, aunque en un estudio retrospectivo se encontró una correlación firme entre los resultados positivos de los estudios citológicos del cuello uterino y la enfermedad endometrial de riesgo alto (es decir, tumor de grado alto e invasión profunda del miometrio). En un estudio prospectivo se encontró una relación estadísticamente significativa entre las características malignas en el estudio citológico y el aumento del riesgo de enfermedad ganglionar.
Factores pronósticos
Los siguientes son los factores pronósticos del cáncer de endometrio:
- Estadio y grado del tumor (incluso diseminación ganglionar extrauterina).
- Estado del receptor hormonal.
Estadio y grado del tumor (incluso diseminación ganglionar extrauterina)
En el cuadro siguiente se subraya el riesgo de metástasis ganglionares de acuerdo con los hallazgos en el momento de la cirugía para la estadificación:
| Grupo pronóstico | Características de la paciente | Riesgo de compromiso ganglionar |
|---|---|---|
| A | Tumores de grado 1 que solo comprometen el endometrio | <5 % |
| No hay indicios de diseminación intraperitoneal | ||
| B | Tumores de grado 2–3 | 5–9 % de los ganglios pélvicos |
| Invasión de <50 % del miometrio | ||
| No hay diseminación intraperitoneal | 4 % de los ganglios paraaórticos | |
| C | Invasión profunda de los músculos | 20–60 % de los ganglios pélvicos |
| Tumores de grado alto | 10–30 % de los ganglios paraaórticos | |
| Diseminación intraperitoneal |
En un estudio del Gynecologic Oncology Group, se relacionaron los parámetros quirúrgico-patológicos y el tratamiento posoperatorio con el intervalo libre de recidiva y el sitio de recidiva. Las características histológicas de grado 3 y la invasión profunda del miometrio en pacientes sin diseminación extrauterina fueron los mayores determinantes de la recidiva. En este estudio, la frecuencia de la recidiva aumentó mucho ante la presencia de lo siguiente:
- Ganglios pélvicos positivos.
- Metástasis anexial.
- Estudio citológico positivo del peritoneo.
- Compromiso del espacio capilar.
- Compromiso del istmo o el cuello uterino.
- Ganglios paraórticos positivos (incluye todos los grados y profundidades de la invasión). De los casos con metástasis en los ganglios aórticos, el 98 % correspondieron a pacientes con ganglios pélvicos positivos, metástasis intraabdominal o invasión tumoral del 33 % (porción más externa) del miometrio.
Cuando el único indicio de diseminación extrauterina es un estudio citológico positivo, la influencia en el desenlace no es clara. El valor del tratamiento dirigido según este hallazgo citológico no está bien fundamentado y algunos datos son contradictorios. Aunque todavía se indica la recolección de muestras citológicas, un resultado positivo no sobrestadifica el cáncer. Antes de considerar terapia posoperatoria adicional, se debe determinar la presencia de otra enfermedad extrauterina.
El compromiso del espacio capilar linfático en el examen histopatológico se correlaciona con la diseminación extrauterina y ganglionar del tumor.
Estado del receptor hormonal
En la evaluación de pacientes con cáncer en estadio I y estadio II, cuando es posible, se incluyen los receptores de progesterona y estrógeno analizados mediante métodos bioquímicos o inmunohistoquímicos.
En un informe se encontró que las concentraciones de progesterona fueron el indicador pronóstico más importante de la supervivencia a 3 años para la enfermedad en estadios clínicos I y II. Las pacientes con concentraciones de progesterona de 100 o más altas tuvieron una tasa de supervivencia sin enfermedad a 3 años de 93 % comparada con 36 % en aquellas con una concentración inferior a 100. Después del ajuste por las concentraciones de los receptores de progesterona, las únicas variables pronósticas importantes fueron el compromiso del cuello uterino y las características citológicas peritoneales.
En otros informes se confirmó la importancia del estado del receptor hormonal como factor pronóstico independiente. Además, se observó que la tinción inmunohistoquímica de tejido embebido en parafina, tanto para los receptores de estrógeno como de progesterona, se correlaciona con el grado y la supervivencia que establece la Fédération Internationale de Gynécologie et d'Obstétrique (FIGO).
Otros factores pronósticos
Otros factores que predicen un pronóstico precario son los siguientes:
- Fracción de fase S alta.
- Aneuploidía.
- Ausencia de PTEN.
- Estado de la mutación de PIK3CA.
- Estado de la mutación de p53.
- Sobreexpresión de Her-2/neu.
- Expresión de un oncogén (por ejemplo, la sobreexpresión del oncogén Her-2/neu se ha relacionado con un pronóstico general precario).
Se publicó una revisión general de factores pronósticos.
References
- American Cancer Society: Cancer Facts and Figures 2023. American Cancer Society, 2023. Available online. Last accessed Dec. 15, 2023.
- Ward KK, Shah NR, Saenz CC, et al.: Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol 126 (2): 176-9, 2012.
- Beral V, Bull D, Reeves G, et al.: Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 365 (9470): 1543-51, 2005 Apr 30-May 6.
- Anderson GL, Limacher M, Assaf AR, et al.: Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291 (14): 1701-12, 2004.
- Furness S, Roberts H, Marjoribanks J, et al.: Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database Syst Rev (2): CD000402, 2009.
- Grady D, Gebretsadik T, Kerlikowske K, et al.: Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol 85 (2): 304-13, 1995.
- Smith DC, Prentice R, Thompson DJ, et al.: Association of exogenous estrogen and endometrial carcinoma. N Engl J Med 293 (23): 1164-7, 1975.
- Mack TM, Pike MC, Henderson BE, et al.: Estrogens and endometrial cancer in a retirement community. N Engl J Med 294 (23): 1262-7, 1976.
- Ziel HK, Finkle WD: Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med 293 (23): 1167-70, 1975.
- Walker AM, Jick H: Cancer of the corpus uteri: increasing incidence in the United States, 1970--1975. Am J Epidemiol 110 (1): 47-51, 1979.
- Gray LA, Christopherson WM, Hoover RN: Estrogens and endometrial carcinoma. Obstet Gynecol 49 (4): 385-9, 1977.
- McDonald TW, Annegers JF, O'Fallon WM, et al.: Exogenous estrogen and endometrial carcinoma: case-control and incidence study. Am J Obstet Gynecol 127 (6): 572-80, 1977.
- Antunes CM, Strolley PD, Rosenshein NB, et al.: Endometrial cancer and estrogen use. Report of a large case-control study. N Engl J Med 300 (1): 9-13, 1979.
- Shapiro S, Kelly JP, Rosenberg L, et al.: Risk of localized and widespread endometrial cancer in relation to recent and discontinued use of conjugated estrogens. N Engl J Med 313 (16): 969-72, 1985.
- Fisher B, Costantino JP, Redmond CK, et al.: Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 86 (7): 527-37, 1994.
- Cummings SR, Eckert S, Krueger KA, et al.: The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 281 (23): 2189-97, 1999.
- DeMichele A, Troxel AB, Berlin JA, et al.: Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol 26 (25): 4151-9, 2008.
- Bergström A, Pisani P, Tenet V, et al.: Overweight as an avoidable cause of cancer in Europe. Int J Cancer 91 (3): 421-30, 2001.
- Aune D, Navarro Rosenblatt DA, Chan DS, et al.: Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol 26 (8): 1635-48, 2015.
- Esposito K, Chiodini P, Capuano A, et al.: Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine 45 (1): 28-36, 2014.
- Troisi R, Potischman N, Hoover RN, et al.: Insulin and endometrial cancer. Am J Epidemiol 146 (6): 476-82, 1997.
- Tsilidis KK, Kasimis JC, Lopez DS, et al.: Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350: g7607, 2015.
- Dossus L, Allen N, Kaaks R, et al.: Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 127 (2): 442-51, 2010.
- Brown SB, Hankinson SE: Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids 99 (Pt A): 8-10, 2015.
- Barry JA, Azizia MM, Hardiman PJ: Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 20 (5): 748-58, 2014 Sep-Oct.
- Win AK, Reece JC, Ryan S: Family history and risk of endometrial cancer: a systematic review and meta-analysis. Obstet Gynecol 125 (1): 89-98, 2015.
- Daniels MS: Genetic testing by cancer site: uterus. Cancer J 18 (4): 338-42, 2012 Jul-Aug.
- Dunlop MG, Farrington SM, Nicholl I, et al.: Population carrier frequency of hMSH2 and hMLH1 mutations. Br J Cancer 83 (12): 1643-5, 2000.
- Lynch HT, Lynch J, Conway T, et al.: Familial aggregation of carcinoma of the endometrium. Am J Obstet Gynecol 171 (1): 24-7, 1994.
- Lu KH, Schorge JO, Rodabaugh KJ, et al.: Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol 25 (33): 5158-64, 2007.
- Widra EA, Dunton CJ, McHugh M, et al.: Endometrial hyperplasia and the risk of carcinoma. Int J Gynecol Cancer 5 (3): 233-235, 1995.
- Jick SS, Walker AM, Jick H: Estrogens, progesterone, and endometrial cancer. Epidemiology 4 (1): 20-4, 1993.
- Jick SS: Combined estrogen and progesterone use and endometrial cancer. Epidemiology 4 (4): 384, 1993.
- Bilezikian JP: Major issues regarding estrogen replacement therapy in postmenopausal women. J Womens Health 3 (4): 273-82, 1994.
- van Leeuwen FE, Benraadt J, Coebergh JW, et al.: Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet 343 (8895): 448-52, 1994.
- DuBeshter B, Warshal DP, Angel C, et al.: Endometrial carcinoma: the relevance of cervical cytology. Obstet Gynecol 77 (3): 458-62, 1991.
- Larson DM, Johnson KK, Reyes CN, et al.: Prognostic significance of malignant cervical cytology in patients with endometrial cancer. Obstet Gynecol 84 (3): 399-403, 1994.
- Takeshima N, Hirai Y, Tanaka N, et al.: Pelvic lymph node metastasis in endometrial cancer with no myometrial invasion. Obstet Gynecol 88 (2): 280-2, 1996.
- Morrow CP, Bundy BN, Kurman RJ, et al.: Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 40 (1): 55-65, 1991.
- Lanciano RM, Corn BW, Schultz DJ, et al.: The justification for a surgical staging system in endometrial carcinoma. Radiother Oncol 28 (3): 189-96, 1993.
- Ambros RA, Kurman RJ: Combined assessment of vascular and myometrial invasion as a model to predict prognosis in stage I endometrioid adenocarcinoma of the uterine corpus. Cancer 69 (6): 1424-31, 1992.
- Turner DA, Gershenson DM, Atkinson N, et al.: The prognostic significance of peritoneal cytology for stage I endometrial cancer. Obstet Gynecol 74 (5): 775-80, 1989.
- Piver MS, Recio FO, Baker TR, et al.: A prospective trial of progesterone therapy for malignant peritoneal cytology in patients with endometrial carcinoma. Gynecol Oncol 47 (3): 373-6, 1992.
- Kadar N, Homesley HD, Malfetano JH: Positive peritoneal cytology is an adverse factor in endometrial carcinoma only if there is other evidence of extrauterine disease. Gynecol Oncol 46 (2): 145-9, 1992.
- Lurain JR: The significance of positive peritoneal cytology in endometrial cancer. Gynecol Oncol 46 (2): 143-4, 1992.
- Lurain JR, Rice BL, Rademaker AW, et al.: Prognostic factors associated with recurrence in clinical stage I adenocarcinoma of the endometrium. Obstet Gynecol 78 (1): 63-9, 1991.
- Garg G, Gao F, Wright JD, et al.: Positive peritoneal cytology is an independent risk-factor in early stage endometrial cancer. Gynecol Oncol 128 (1): 77-82, 2013.
- Hanson MB, van Nagell JR, Powell DE, et al.: The prognostic significance of lymph-vascular space invasion in stage I endometrial cancer. Cancer 55 (8): 1753-7, 1985.
- Carcangiu ML, Chambers JT, Voynick IM, et al.: Immunohistochemical evaluation of estrogen and progesterone receptor content in 183 patients with endometrial carcinoma. Part I: Clinical and histologic correlations. Am J Clin Pathol 94 (3): 247-54, 1990.
- Chambers JT, Carcangiu ML, Voynick IM, et al.: Immunohistochemical evaluation of estrogen and progesterone receptor content in 183 patients with endometrial carcinoma. Part II: Correlation between biochemical and immunohistochemical methods and survival. Am J Clin Pathol 94 (3): 255-60, 1990.
- Gurpide E: Endometrial cancer: biochemical and clinical correlates. J Natl Cancer Inst 83 (6): 405-16, 1991.
- Ingram SS, Rosenman J, Heath R, et al.: The predictive value of progesterone receptor levels in endometrial cancer. Int J Radiat Oncol Biol Phys 17 (1): 21-7, 1989.
- Creasman WT: Prognostic significance of hormone receptors in endometrial cancer. Cancer 71 (4 Suppl): 1467-70, 1993.
- Friberg LG, Norén H, Delle U: Prognostic value of DNA ploidy and S-phase fraction in endometrial cancer stage I and II: a prospective 5-year survival study. Gynecol Oncol 53 (1): 64-9, 1994.
- Hetzel DJ, Wilson TO, Keeney GL, et al.: HER-2/neu expression: a major prognostic factor in endometrial cancer. Gynecol Oncol 47 (2): 179-85, 1992.
- Binder PS, Mutch DG: Update on prognostic markers for endometrial cancer. Womens Health (Lond Engl) 10 (3): 277-88, 2014.
Clasificación celular del cáncer de endometrio
Los cánceres de endometrio se clasifican en los siguientes tipos:
- Tipo 1: surge de una hiperplasia atípica compleja y se vincula en su patogenia con estimulación estrogénica sin oposición.
- Tipo 2: surge en un endometrio atrófico y no se vincula con patogenia hormonal.
El tipo más común de cáncer de endometrio es el adenocarcinoma endometrioide.
La frecuencia de los tipos de células de cáncer de endometrio es la siguiente:
- Adenocarcinoma endometrioide (75 %); compuesto por elementos malignos de epitelio glandular; no es infrecuente una mezcla de metaplasia escamosa.
- Adenocarcinoma ciliado.
- Adenocarcinoma secretor.
- Adenocarcinomas papilar y velloglandular con características histológicas similares a las observadas en el ovario y la trompa de Falopio. El pronóstico es más precario para estos tumores.
- Adenocarcinoma con diferenciación escamosa.
- Adenoacantoma.
- Células adenoescamosas con elementos epiteliales glandulares y de células escamosas malignos.
- Mixto; definido por la presencia de dos tipos de células carcinomatosas, con un componente más pequeño de por lo menos 10 % del total (10 %).
- Seroso papilar uterino (<10 %).
- Células claras (4 %), similares desde el punto de vista histológico a aquellas observadas en el ovario y la trompa de Falopio. El pronóstico de los tumores de células claras es más precario.
- Carcinosarcoma (3 %), también conocido como tumor mesodérmico mixto maligno, presenta tanto elementos carcinomatosos como sarcomatosos. En el pasado, este tumor se categorizaba como subtipo de los sarcomas uterinos; sin embargo, la evidencia reciente indica que se origina como adenocarcinoma sometido a una diferenciación de sus elementos sarcomatosos.
- Mucinoso (10 %)
- Células escamosas (<1 %).
- Indiferenciado (<1 %).
Subgrupos moleculares
Las mutaciones en PTEN son más comunes en los cánceres de endometrio de tipo 1; las sobreexpresiones de p53 y Her-2/neu son más comunes en los cánceres de endometrio de tipo 2; sin embargo, hay cierta superposición.
En la visualización genética completa del Cancer Genome Atlas de cientos de cánceres de endometrio, se identificaron cuatro subtipos para caracterizar aún más los cánceres de endometrio:
- Tumores ultramutados POLE. Este subtipo tiene importancia clínica y se evita la administración de terapias adyuvantes.
- Inestabilidad de microsatélites con hipermutación.
- Número bajo de copias.
- Número alto de copias.
Estas categorías se pueden usar para estratificar a las pacientes en categorías pronósticas de riesgo bajo y alto. Una modificación de los métodos del Cancer Genome Atlas en pruebas más accesibles también tuvo éxito en la discriminación de cánceres en categoría pronósticas importantes. Sin embargo, una combinación de factores de riesgo previamente conocidos con datos genéticos fue más eficaz para determinar categorías pronósticas.
References
- Gusberg SB: Virulence factors in endometrial cancer. Cancer 71 (4 Suppl): 1464-6, 1993.
- Zaino RJ, Kurman R, Herbold D, et al.: The significance of squamous differentiation in endometrial carcinoma. Data from a Gynecologic Oncology Group study. Cancer 68 (10): 2293-302, 1991.
- Kandoth C, Schultz N, Cherniack AD, et al.: Integrated genomic characterization of endometrial carcinoma. Nature 497 (7447): 67-73, 2013.
- Talhouk A, McConechy MK, Leung S, et al.: A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 113 (2): 299-310, 2015.
Información sobre los estadios del cáncer de endometrio
El modelo de diseminación del cáncer de endometrio depende, en parte, del grado de diferenciación celular. Los tumores bien diferenciados tienden a limitar su diseminación a la superficie del endometrio; la invasión del miometrio es menos común. La invasión del miometrio se presenta con más frecuencia en pacientes con tumores pobremente diferenciados y, a menudo, presagia el compromiso de los ganglios linfáticos y las metástasis a distancia.
La diseminación metastásica tiene un modelo característico. La diseminación regional a los ganglios pélvicos y paraaórticos es común. La diseminación a distancia por lo común compromete los siguientes sitios:
- Pulmones.
- Ganglios inguinales y supraclaviculares.
- Hígado.
- Huesos.
- Encéfalo.
- Vagina.
Estadificación de la Fédération Internationale de Gynécologie et d’Obstétrique
La Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) junto con el American Joint Committee on Cancer (AJCC) diseñaron la estadificación para definir el cáncer de endometrio. El sistema FIGO es el sistema de estadificación que se usa con mayor frecuencia para estadificar el cáncer de endometrio.
Los estadios FIGO I a IV se subdividen según el grado histológico del tumor (G); por ejemplo, estadio IB G2. Los carcinosarcomas, que antes se designaban como sarcomas, ahora se consideran adenocarcinomas pobremente diferenciados; por lo tanto, se incluyen en este sistema.
| Estadio | Descripción | Ilustración |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d’Obstétrique. | ||
| aAdaptado del FIGO Committee on Gynecologic Oncology. | ||
| bG1, G2 o G3 (G = grado). | ||
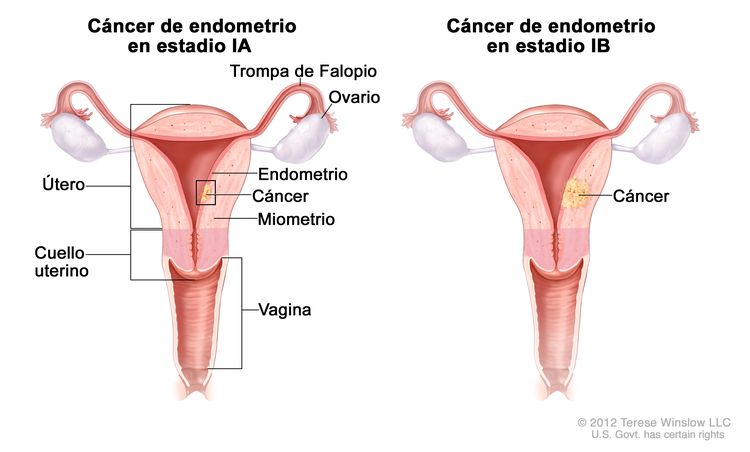
| Ib | Tumor confinado en el cuerpo del útero. |
|
| IAb | Ausencia de invasión al endometrio o invasión a menos de la mitad del miometrio. | |
| IBb | Invasión a la mitad o más de la mitad del miometrio. | |
| Estadio | Descripción | Ilustración |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d’Obstétrique. | ||
| aAdaptado del FIGO Committee on Gynecologic Oncology. | ||
| bG1, G2 o G3 (G = grado). | ||
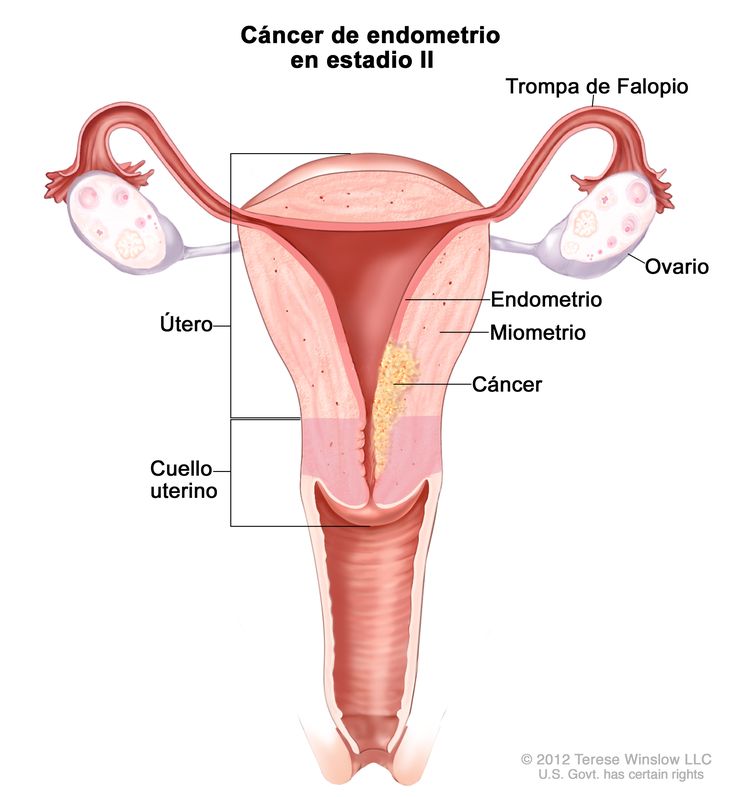
| cEl compromiso endocervical glandular se considera estadio I; ya no se considera estadio II. | ||
| IIb | Tumor con invasión del estroma del cuello uterino, pero sin diseminación fuera del útero.c |
|
| Estadio | Descripción | Ilustración |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d’Obstétrique. | ||
| aAdaptado del FIGO Committee on Gynecologic Oncology. | ||
| bG1, G2 o G3 (G = grado). | ||
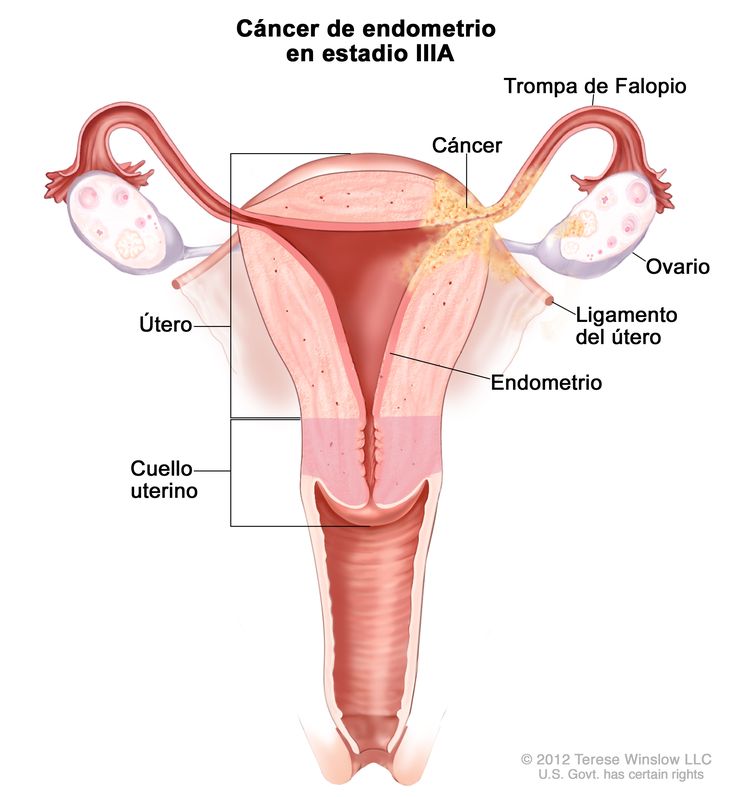
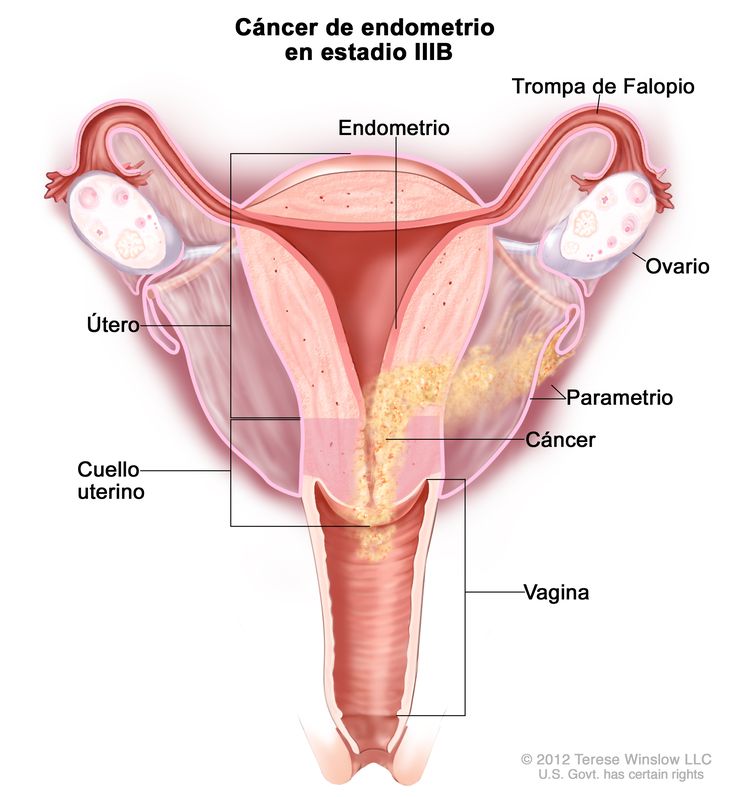
| cSe deben notificar los estudios citológicos con resultado positivo por separado sin cambiar el estadio. | ||
| IIIb | Diseminación local o regional del tumor. | |
| IIIAb | Tumor con invasión de la serosa del cuerpo del útero o los anexos uterinos.c |
|
| IIIBb | Compromiso de la vagina o el parametrio.c |
|
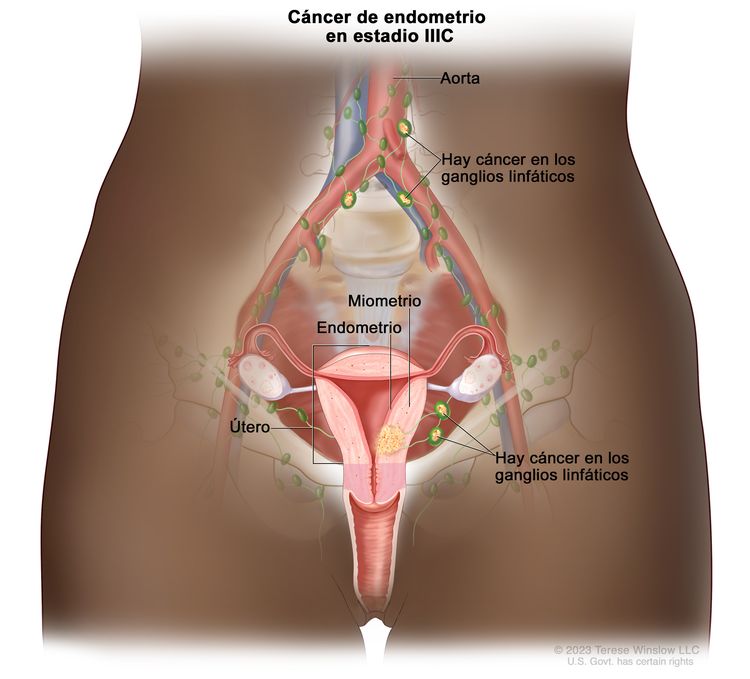
| IIICb | Metástasis en los ganglios linfáticos pélvicos o paraaórticos.c |
|
| IIIC1b | Compromiso de ganglios pélvicos. | |
| IIIC2b | Compromiso de ganglios linfáticos paraaórticos con compromiso de ganglios linfáticos pélvicos o sin este. | |
| Estadio | Descripción | Ilustración |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d’Obstétrique. | ||
| aAdaptado del FIGO Committee on Gynecologic Oncology. | ||
| bG1, G2 o G3 (G = grado). | ||
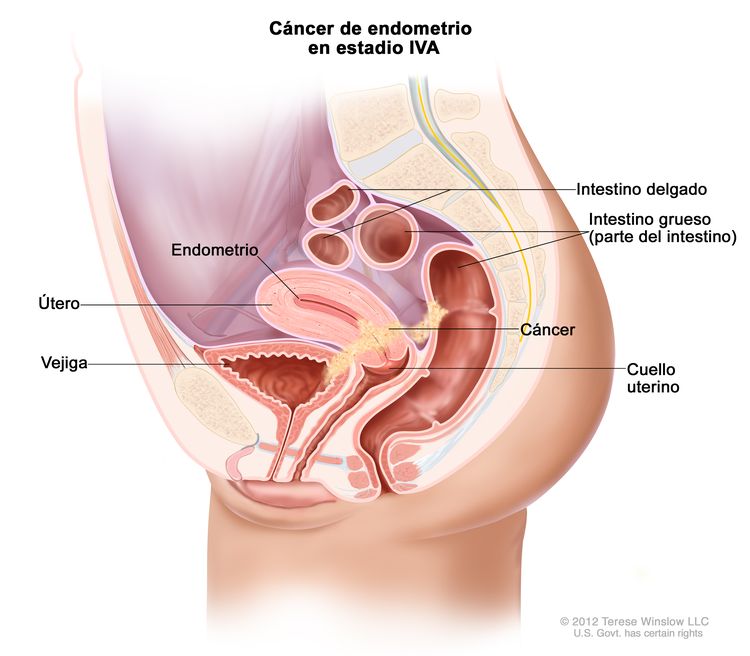
| IVb | Tumor con invasión de la vejiga o la mucosa intestinal, o metástasis a distancia. | |
| IVAb | Invasión tumoral de la vejiga o la mucosa intestinal. |
|
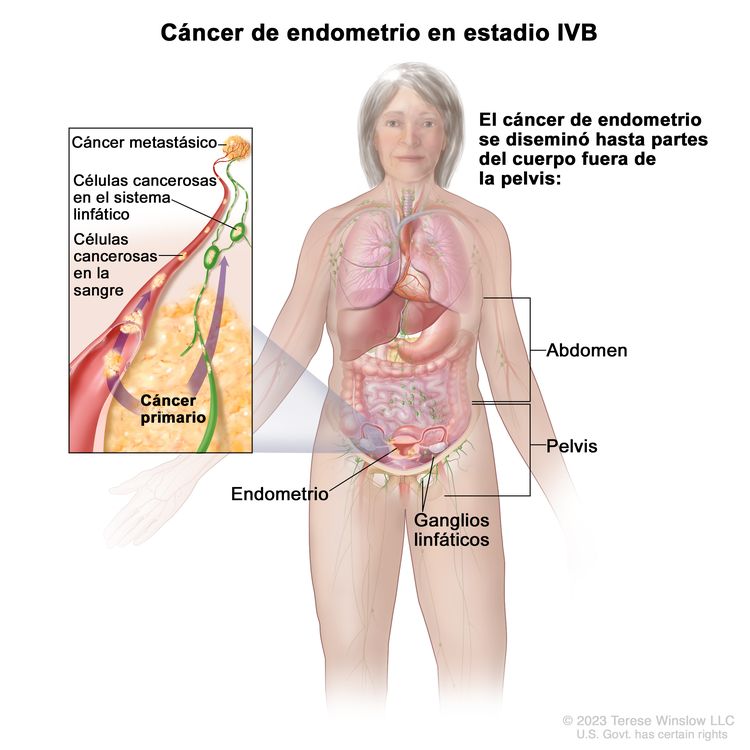
| IVBb | Metástasis a distancia, incluso metástasis intraabdominales o a los ganglios linfáticos inguinales. |
|
References
- Hendrickson M, Ross J, Eifel PJ, et al.: Adenocarcinoma of the endometrium: analysis of 256 cases with carcinoma limited to the uterine corpus. Pathology review and analysis of prognostic variables. Gynecol Oncol 13 (3): 373-92, 1982.
- Nori D, Hilaris BS, Tome M, et al.: Combined surgery and radiation in endometrial carcinoma: an analysis of prognostic factors. Int J Radiat Oncol Biol Phys 13 (4): 489-97, 1987.
- Koskas M, Amant F, Mirza MR, et al.: Cancer of the corpus uteri: 2021 update. Int J Gynaecol Obstet 155 (Suppl 1): 45-60, 2021.
- Corpus uteri – carcinoma and carcinosarcoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 661-69.
Aspectos generales de las opciones de tratamiento del cáncer de endometrio
El grado de diferenciación tumoral tiene un efecto importante en la evolución natural de esta enfermedad y en la selección del tratamiento.
Las pacientes de cáncer de endometrio que tienen la enfermedad localizada se suelen curar. Los mejores resultados se obtienen con cualquiera de los dos tratamientos estándar:
- Histerectomía con salpingooforectomía bilateral.
- Histerectomía con salpingooforectomía bilateral y radioterapia adyuvante (cuando hay invasión profunda del músculo del miometrio [más de 50 % del miometrio] o cuando hay un tumor de grado 3 con invasión del miometrio).
Las pacientes con metástasis regionales y a distancia se curan con muy poca frecuencia, aunque en ocasiones responden a la terapia hormonal estándar.
En varios ensayos aleatorizados se evaluaron sustancias progestacionales para administrarlas como terapia adyuvante. En un metanálisis del grupo Cochrane se confirmó que no hay un beneficio clínico de los progestágenos adyuvantes en el entorno de la enfermedad en estadio clínico I.[Nivel de evidencia A1]
Las opciones de tratamiento para cada estadio del cáncer de endometrio se presentan en el Cuadro 6.
| Estadio (definiciones FIGO de estadificación) | Opciones de tratamiento | |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d’Obstétrique. | ||
| Cáncer de endometrio en estadio I y estadio II | Grados 1 y 2 | Cirugía con muestreo de ganglios linfáticos o sin este |
| Braquiterapia vaginal posoperatoria | ||
| Radioterapia sola | ||
| Grado 3 (incluye carcinoma seroso y de células claras, y carcinosarcoma) | Cirugía | |
| Quimioterapia posoperatoria con radioterapia o sin esta | ||
| Cáncer de endometrio en estadio III, estadio IV y recidivante | Enfermedad operable | Cirugía seguida de quimioterapia o radioterapia |
| Enfermedad inoperable | Quimioterapia y radioterapia | |
| Enfermedad inoperable en una paciente que no es apta para radioterapia | Terapia hormonal | |
| Terapia biológica | ||
References
- Martin-Hirsch PP, Bryant A, Keep SL, et al.: Adjuvant progestagens for endometrial cancer. Cochrane Database Syst Rev (6): CD001040, 2011.
Tratamiento del cáncer de endometrio en estadio I y estadio II
Opciones de tratamiento estándar del cáncer de endometrio en estadio I y estadio II
El tratamiento del cáncer de endometrio en estadio I y estadio II depende del grado y el tipo histológico.
En el sistema actual de estadificación de la Fédération Internationale de Gynécologie et d’Obstétrique (FIGO), el estadio II describe un tumor que invade el estroma del cuello uterino; esto equivale al estadio IIB del sistema anterior. En casi todos los ensayos aleatorizados de cáncer en estadio temprano se excluyó a pacientes en estadio IIB. Como resultado, hay pocos datos de calidad para tomar decisiones clínicas en el caso de pacientes en estadio II.
Características histológicas de riesgo bajo:
Los tumores de grados 1 y 2 se consideran de riesgo bajo a menos que sean del subtipo seroso o de células claras.
Las opciones de tratamiento estándar para pacientes de cáncer de endometrio de subtipos histológicos de riesgo bajo son las siguientes:
- Cirugía: histerectomía con salpingooforectomía bilateral y posible disección de ganglios linfáticos.
- Braquiterapia vaginal posoperatoria.
- Radioterapia sola.
La mayoría de las pacientes evolucionan bien con cirugía sola. Un subconjunto de pacientes con enfermedad en estadio I tienen riesgo alto de recidiva y son aptas para recibir terapia adyuvante.
Características histológicas de riesgo alto:
Se consideran de riesgo alto los tumores de grado 3, cualesquiera sean sus características histológicas, así como todos los tumores serosos y de células claras, o los carcinosarcomas.
Las opciones de tratamiento para pacientes de cáncer de endometrio en estadio I o estadio II con subtipos histológicos de riesgo alto son las siguientes:
- Cirugía: histerectomía con salpingooforectomía bilateral, y disección de ganglios linfáticos pélvicos y paraaórticos.
- Quimioterapia posoperatoria con radioterapia o sin esta.
Las pacientes con características histológicas serosas o de células claras tienen tasas más altas de recidiva que las pacientes con otros carcinomas endometrioides en estadio I o estadio II. Se publicaron los desenlaces en series de casos institucionales en las que se utilizó un régimen de carboplatino adyuvante con paclitaxel que, en ocasiones, incluyó radioterapia para este subtipo histológico. Estos constituyen la base de las directrices de tratamiento.
Los carcinosarcomas se evaluaron en ensayos clínicos por separado o con otros sarcomas debido a su clasificación anterior en este grupo. En un estudio no aleatorizado del Gynecologic Oncology Group (GOG) de pacientes con carcinosarcomas en estadio I o II, las pacientes sometidas a radioterapia pélvica presentaron una reducción importante de recidivas dentro del campo de radioterapia, pero no mejoró la supervivencia. En un estudio no aleatorizado con predominio de pacientes de carcinosarcoma, pareció observarse un beneficio de la terapia adyuvante con cisplatino y doxorrubicina.
Cirugía
En el caso de compromiso del cuello uterino, las opciones de tratamiento son las siguientes:
- Histerectomía estándar con salpingooforectomía bilateral seguidas de radioterapia adyuvante.
- Histerectomía radical.
- Disección de ganglios linfáticos pélvicos y paraaórticos.
En una revisión de una sola institución se indica que la histerectomía radical es más beneficiosa que la histerectomía estándar en casos de compromiso tumoral del cuello uterino.
Cirugía con muestreo de ganglios linfáticos o sin este
En el cuadro siguiente se subraya el riesgo de metástasis ganglionares de acuerdo con los hallazgos en el momento de la cirugía para la estadificación:
| Grupo pronóstico | Características de la paciente | Riesgo de compromiso ganglionar |
|---|---|---|
| A | Tumores de grado 1 que solo comprometen el endometrio | <5 % |
| No hay indicios de diseminación intraperitoneal | ||
| B | Tumores de grado 2–3 | 5–9 % de los ganglios pélvicos |
| Invasión de <50 % del miometrio | ||
| No hay diseminación intraperitoneal | 4 % de los ganglios paraaórticos | |
| C | Invasión profunda de los músculos | 20–60 % de los ganglios pélvicos |
| Tumores de grado alto | 10–30 % de los ganglios paraaórticos | |
| Diseminación intraperitoneal |
La disección de ganglios linfáticos es de utilidad limitada para las pacientes del grupo A. Por el contrario, la disección completa de ganglios linfáticos pélvicos y paraaórticos es importante para pacientes del grupo C debido a la probabilidad de hallazgos positivos. La dificultad reside en determinar el manejo adecuado de las pacientes del grupo B.
Hay varios abordajes quirúrgicos aceptados para pacientes de cáncer de endometrio que se presume que son de estadio I con riesgo intermedio de diseminación linfática.
Tanto los datos retrospectivos como los prospectivos apoyan la estratificación de pacientes de cáncer de endometrio que se presume que son de estadio I en dos grupos según las siguientes características:
- Riesgo bajo: tumor bien diferenciado o moderadamente diferenciado, o profundidad de la invasión del miometrio de menos de 50 % y el tumor mide menos de 2 cm.
- Riesgo alto: tumor pobremente diferenciado o profundidad de la invasión del miometrio de 50 % o más, y el tumor mide 2 cm o más.
Evidencia (disección de ganglios linfáticos):
- En estos estudios, las pacientes de cáncer de riesgo bajo presentaban un riesgo de metástasis ganglionares bajo que permitió omitir el muestreo de ganglios linfáticos. Para las pacientes que satisfacían los criterios de riesgo alto, se indicó una disección de ganglios linfáticos pélvicos y paraaórticos completa.
- Una estrategia alternativa es usar la disección de ganglios linfáticos centinela de pacientes con presumible cáncer de endometrio en estadio I. Aunque esta estrategia es de amplia aceptación en varios centros académicos, no hay un ensayo prospectivo multicéntrico para determinar la tasa de resultados negativos falsos de este protocolo. En los casos en los que se identifican células tumorales aisladas con el abordaje del ganglio linfático centinela, no está claro si se necesita tratamiento.
- El estándar para las pacientes con características histológicas de riesgo alto (carcinosarcoma seroso y de células claras, o tumores indiferenciados) es la histerectomía y la salpingooforectomía bilateral con disección de ganglios linfáticos pélvicos y paraaórticos.
- La laparotomía fue el abordaje estándar. Sin embargo, en la actualidad se favorece la laparoscopia debido a la mejora en la recuperación posoperatoria de las pacientes sin efectos importantes en los desenlaces.
Evidencia (tratamiento o estadificación quirúrgica mediante laparoscopia vs. laparotomía):
- Para las pacientes de cáncer de endometrio en estadio temprano, en varios ensayos aleatorizados se comparó la histerectomía laparoscópica total (HLT) con el procedimiento estándar, histerectomía abdominal total (HAT). La factibilidad del abordaje laparoscópico ha sido confirmada, pero este abordaje exige un período operatorio más largo. La HLT tiene un perfil de episodios adversos mejor o similar , y una hospitalización más breve que la HAT.
- La HLT se relaciona con menos dolor y una reanudación más rápida de las actividades diarias, aunque en un estudio se encontró que, si bien la mayor parte de las ganancias en la calidad de vida favorecieron la laparoscopia en el período posquirúrgico de 6 semanas, ya no fueron significativas a los 6 meses.
- En un ensayo del GOP (GOG-LAP2), se asignó al azar a 2616 pacientes con enfermedad en estadios clínicos I y IIA en una proporción 2:1 a una estadificación quirúrgica exhaustiva con laparoscopia o laparotomía.[Nivel de evidencia A1]
El criterio primario de valoración fue el tiempo hasta la recidiva, y la ausencia de inferioridad se definió como una diferencia en la tasa de recidiva a 3 años menor de 5,3 % entre los dos grupos.
- La tasa de recidiva a 3 años fue de 10,24 % para las pacientes del grupo de laparotomía y de 11,39 % para las pacientes del grupo de laparoscopia, con una diferencia calculada entre los grupos de 1,14 % (límite inferior de 90 %, -1278; límite superior de 95 %, 3996).
- Aunque esta diferencia fue más baja que el límite preespecificado, no se reunieron los requisitos estadísticos de ausencia de inferioridad debido a un número de recidivas menor del previsto en ambos grupos.
- La tasa de supervivencia general (SG) a 5 años fue de 89,8 % en ambos grupos.
- La tasa de recidiva a 3 años fue de 10,24 % para las pacientes del grupo de laparotomía y de 11,39 % para las pacientes del grupo de laparoscopia, con una diferencia calculada entre los grupos de 1,14 % (límite inferior de 90 %, -1278; límite superior de 95 %, 3996).
- En una revisión de Cochrane sobre el uso de la estadificación laparoscópica, se incluyeron cuatro ensayos controlados aleatorizados que notificaron SG y supervivencia sin progresión (SSP). Noventa por ciento de las pacientes pertenecían al ensayo GOG-LAP2.[ Nivel de evidencia A1]
- En general, la laparoscopia y la laparotomía se vincularon con tasas similares de SG y SSP.
En los análisis futuros tal vez sea posible determinar si hay subgrupos de pacientes que presentan una disminución clínicamente significativa cuando se utiliza la estadificación laparoscópica.[Nivel de evidencia B1]
Braquiterapia vaginal posoperatoria
Si bien la radioterapia adyuvante reducirá la incidencia de recidivas locales y locorregionales, no se comprobó una mejora de la supervivencia y los efectos tóxicos son peores con la radioterapia. La braquiterapia en la cúpula vaginal se vincula con menor morbilidad relacionada con la radiación que la radioterapia de haz externo (RHE) y mostró ser equivalente a la RHE en el corto plazo para pacientes con enfermedad en estadio I. Sin embargo, en el seguimiento a largo plazo de un ensayo aleatorizado en el que se comparó el uso combinado de la RHE y la braquiterapia vaginal (BTV) con la BTV sola, se encontró una disminución de la SG y un aumento de los efectos tóxicos en el grupo que recibió RHE además de BTV.
Evidencia (braquiterapia vaginal):
- En los resultados de dos ensayos aleatorios en los que se usó radioterapia adyuvante para pacientes con enfermedad en estadio I, no se observó una mejora de la supervivencia pero se observó una reducción de la recidiva locorregional (3–4 % en el grupo de radioterapia vs. 12–14 % en el grupo de control después de mediana de seguimiento de 5–6 años; P< 0,001), con un aumento de los efectos secundarios.[Nivel de evidencia B1]
- Los resultados de un estudio realizado por el Danish Endometrial Cancer Group indican que la ausencia de radioterapia no mejora la supervivencia de las pacientes con enfermedad de riesgo intermedio en estadio I (grados 1 y 2 con >50 % de invasión del miometrio, o grado 3 con <50 % de invasión miometrial).
- En el ensayo PORTEC-2 (NCT00411138), se asignó al azar a pacientes de cáncer de endometrio en estadio I que no se sometieron a disección de ganglios linfáticos a recibir BTV o RHE; el criterio primario de valoración fue la prevención de la recidiva vaginal.[Nivel de evidencia A1]
- A los 5 años, no hubo ninguna diferencia en las tasas de recidiva vaginal, recidiva locorregional, SSP o SG (84,8 % intervalo de confianza [IC] 95 %, 79,3–90,3 % para la BTV vs. 79,6 % [IC 95 %, 71,2–88,0 %] para la RHE; P = 0,57).
- En el grupo de BTV se presentaron considerablemente menos efectos tóxicos gastrointestinales y mejoró la calidad de vida; esto hizo que la BTV se convirtiera en la opción preferida de tratamiento adyuvante para las pacientes con enfermedad en estadio I.
- Entre 1968 y 1974 (antes del inicio de la estadificación quirúrgica de FIGO), en el ensayo Norwegian Radium Hospital se asignó al azar a tratamiento a 568 pacientes de cáncer de endometrio en estadio clínico I.[Nivel de evidencia A1]. Después de la histerectomía y la salpingooforectomía bilateral, las pacientes se asignaron al azar para recibir RHE y BTV, o BTV sola.
- En un informe actualizado en el que se presentaron datos de más de 20 años de seguimiento, no se observó diferencias de SG entre los grupos de tratamiento. La mediana de SG fue de 20,5 años en el grupo de RHE y BTV, y de 20,48 en el grupo de BTV sola (P = 0,186). Todas las mujeres presentaron un aumento de riesgo de cánceres secundarios después de la RHE (cociente de riesgos instantáneos [CRI], 1,42; IC 95 %, 1,01–2,0).
- En un análisis posterior de subconjuntos de las mujeres menores de 60 años en el momento de la inscripción en el ensayo, se observó un aumento de mortalidad en el grupo de RHE (CRI, 1,36; IC 95 %, 1,06–1,76). Además, el riesgo de cánceres secundarios se duplicó en este grupo (CRI, 2,02; IC 95%, 1,3–3,15).
Radioterapia posoperatoria
Si el cuello uterino no está comprometido desde el punto de vista clínico, pero se observa diseminación al cuello uterino en el análisis patológico posoperatorio, se considera la administración de radioterapia.[Nivel de evidencia A1]
Radioterapia sola
Es posible que las pacientes con contraindicaciones médicas para la cirugía se traten con radioterapia sola, pero a veces esto disminuye las tasas de curación que se logran con la cirugía.
Opciones de tratamiento en evaluación clínica del cáncer de endometrio en estadio I y estadio II
- En el ensayo GOG-0249 [NCT00807768], se comparó el uso combinado de carboplatino y paclitaxel adyuvantes además de la braquiterapia en la cúpula vaginal versus RHE pélvica adyuvante para pacientes de cáncer de endometrio con enfermedad en estadio I o estadio II. No se aceptan más inscripciones en el estudio y los hallazgos preliminares no demostraron una diferencia significativa entre los dos grupos de tratamiento.
- En el ensayo GOG-0261 (NCT00954174), se comparó el paclitaxel y carboplatino con paclitaxel e ifosfamida en pacientes de cáncer de útero, ovario, de trompa de Falopio o de cavidad peritoneal en estadios I a IV recién diagnosticadas.
Ensayos clínicos en curso
Realizar una búsqueda avanzada en inglés de los ensayos clínicos sobre cáncer auspiciados por el NCI que ahora aceptan pacientes. La búsqueda se puede simplificar por ubicación del ensayo, tipo de tratamiento, nombre del fármaco y otros criterios. También se dispone de información general sobre los ensayos clínicos.
References
- Kiess AP, Damast S, Makker V, et al.: Five-year outcomes of adjuvant carboplatin/paclitaxel chemotherapy and intravaginal radiation for stage I-II papillary serous endometrial cancer. Gynecol Oncol 127 (2): 321-5, 2012.
- Boruta DM, Gehrig PA, Fader AN, et al.: Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol 115 (1): 142-53, 2009.
- Huh WK, Powell M, Leath CA, et al.: Uterine papillary serous carcinoma: comparisons of outcomes in surgical Stage I patients with and without adjuvant therapy. Gynecol Oncol 91 (3): 470-5, 2003.
- Fader AN, Drake RD, O'Malley DM, et al.: Platinum/taxane-based chemotherapy with or without radiation therapy favorably impacts survival outcomes in stage I uterine papillary serous carcinoma. Cancer 115 (10): 2119-27, 2009.
- Kelly MG, O'malley DM, Hui P, et al.: Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol 98 (3): 353-9, 2005.
- Havrilesky LJ, Secord AA, Bae-Jump V, et al.: Outcomes in surgical stage I uterine papillary serous carcinoma. Gynecol Oncol 105 (3): 677-82, 2007.
- Dietrich CS, Modesitt SC, DePriest PD, et al.: The efficacy of adjuvant platinum-based chemotherapy in Stage I uterine papillary serous carcinoma (UPSC). Gynecol Oncol 99 (3): 557-63, 2005.
- Townamchai K, Berkowitz R, Bhagwat M, et al.: Vaginal brachytherapy for early stage uterine papillary serous and clear cell endometrial cancer. Gynecol Oncol 129 (1): 18-21, 2013.
- Barney BM, Petersen IA, Mariani A, et al.: The role of vaginal brachytherapy in the treatment of surgical stage I papillary serous or clear cell endometrial cancer. Int J Radiat Oncol Biol Phys 85 (1): 109-15, 2013.
- Hornback NB, Omura G, Major FJ: Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys 12 (12): 2127-30, 1986.
- Peters WA, Rivkin SE, Smith MR, et al.: Cisplatin and adriamycin combination chemotherapy for uterine stromal sarcomas and mixed mesodermal tumors. Gynecol Oncol 34 (3): 323-7, 1989.
- Ayhan A, Taskiran C, Celik C, et al.: The long-term survival of women with surgical stage II endometrioid type endometrial cancer. Gynecol Oncol 93 (1): 9-13, 2004.
- Eltabbakh GH, Moore AD: Survival of women with surgical stage II endometrial cancer. Gynecol Oncol 74 (1): 80-5, 1999.
- Orezzoli JP, Sioletic S, Olawaiye A, et al.: Stage II endometrioid adenocarcinoma of the endometrium: clinical implications of cervical stromal invasion. Gynecol Oncol 113 (3): 316-23, 2009.
- Walker JL, Piedmonte MR, Spirtos NM, et al.: Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol 27 (32): 5331-6, 2009.
- Mariani A, Dowdy SC, Cliby WA, et al.: Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol 109 (1): 11-8, 2008.
- Mariani A, Webb MJ, Keeney GL, et al.: Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol 182 (6): 1506-19, 2000.
- Barlin JN, Khoury-Collado F, Kim CH, et al.: The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol 125 (3): 531-5, 2012.
- Janda M, Gebski V, Brand A, et al.: Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet Oncol 11 (8): 772-80, 2010.
- Mourits MJ, Bijen CB, Arts HJ, et al.: Safety of laparoscopy versus laparotomy in early-stage endometrial cancer: a randomised trial. Lancet Oncol 11 (8): 763-71, 2010.
- Kornblith AB, Huang HQ, Walker JL, et al.: Quality of life of patients with endometrial cancer undergoing laparoscopic international federation of gynecology and obstetrics staging compared with laparotomy: a Gynecologic Oncology Group study. J Clin Oncol 27 (32): 5337-42, 2009.
- Walker JL, Piedmonte MR, Spirtos NM, et al.: Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol 30 (7): 695-700, 2012.
- Galaal K, Bryant A, Fisher AD, et al.: Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev 9: CD006655, 2012.
- Aalders J, Abeler V, Kolstad P, et al.: Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol 56 (4): 419-27, 1980.
- Morrow CP, Bundy BN, Kurman RJ, et al.: Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 40 (1): 55-65, 1991.
- Marchetti DL, Caglar H, Driscoll DL, et al.: Pelvic radiation in stage I endometrial adenocarcinoma with high-risk attributes. Gynecol Oncol 37 (1): 51-4, 1990.
- Creutzberg CL, van Putten WL, Koper PC, et al.: Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 355 (9213): 1404-11, 2000.
- Kong A, Johnson N, Kitchener HC, et al.: Adjuvant radiotherapy for stage I endometrial cancer: an updated Cochrane systematic review and meta-analysis. J Natl Cancer Inst 104 (21): 1625-34, 2012.
- Nout RA, Smit VT, Putter H, et al.: Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet 375 (9717): 816-23, 2010.
- Onsrud M, Cvancarova M, Hellebust TP, et al.: Long-term outcomes after pelvic radiation for early-stage endometrial cancer. J Clin Oncol 31 (31): 3951-6, 2013.
- Keys HM, Roberts JA, Brunetto VL, et al.: A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 92 (3): 744-51, 2004.
- Scholten AN, van Putten WL, Beerman H, et al.: Postoperative radiotherapy for Stage 1 endometrial carcinoma: long-term outcome of the randomized PORTEC trial with central pathology review. Int J Radiat Oncol Biol Phys 63 (3): 834-8, 2005.
- Bertelsen K, Ortoft G, Hansen ES: Survival of Danish patients with endometrial cancer in the intermediate-risk group not given postoperative radiotherapy: the Danish Endometrial Cancer Study (DEMCA). Int J Gynecol Cancer 21 (7): 1191-9, 2011.
- Nout RA, Putter H, Jürgenliemk-Schulz IM, et al.: Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur J Cancer 48 (11): 1638-48, 2012.
- Eltabbakh GH, Piver MS, Hempling RE, et al.: Excellent long-term survival and absence of vaginal recurrences in 332 patients with low-risk stage I endometrial adenocarcinoma treated with hysterectomy and vaginal brachytherapy without formal staging lymph node sampling: report of a prospective trial. Int J Radiat Oncol Biol Phys 38 (2): 373-80, 1997.
- Stokes S, Bedwinek J, Kao MS, et al.: Treatment of stage I adenocarcinoma of the endometrium by hysterectomy and adjuvant irradiation: a retrospective analysis of 304 patients. Int J Radiat Oncol Biol Phys 12 (3): 339-44, 1986.
- Grigsby PW, Kuske RR, Perez CA, et al.: Medically inoperable stage I adenocarcinoma of the endometrium treated with radiotherapy alone. Int J Radiat Oncol Biol Phys 13 (4): 483-8, 1987.
Tratamiento del cáncer de endometrio en estadio III, estadio IV y recidivante
Opciones de tratamiento estándar del cáncer de endometrio en estadio III, estadio IV y recidivante
Las opciones de tratamiento estándar del cáncer de endometrio en estadio III, estadio IV y recidivante son las siguientes:
- Cirugía seguida de quimioterapia o radioterapia.
- Quimioterapia y radioterapia.
- Terapia hormonal.
- Terapia biológica.
El tratamiento de las pacientes con cáncer de endometrio en estadio IV se determina según el sitio de la enfermedad metastásica y los síntomas relacionados con los sitios de la enfermedad.
Cirugía seguida de quimioterapia o radioterapia
En general, las pacientes de cáncer de endometrio en estadio III o estadio IV se tratan con cirugía seguida de quimioterapia, radioterapia o ambas. Si bien hay estudios de observación que respaldan la cirugía de citorreducción máxima para las pacientes con enfermedad en estadio IV, estas conclusiones se deben interpretar con cautela debido al número pequeño de casos y los posibles sesgos de selección.
Durante muchos años, la radioterapia fue el tratamiento adyuvante estándar para las pacientes de cáncer de endometrio. Sin embargo, en varios estudios aleatorizados se confirmó una mejora de la supervivencia con la quimioterapia adyuvante en lugar de la radioterapia.
Tradicionalmente, la doxorrubicina fue el fármaco más activo utilizado contra el cáncer, con respuestas útiles pero temporarias obtenidas en por lo menos 33 % de las pacientes con enfermedad recidivante. El paclitaxel, combinado con quimioterapia con derivados del platino o como fármaco único también tiene un efecto importante sobre el cáncer.
Evidencia (cirugía seguida de quimioterapia o radioterapia):
- En varios ensayos aleatorizados realizados por el Gynecologic Oncology Group (GOG), se empleó la conocida actividad tumoral de la doxorrubicina.
- La adición de cisplatino a la doxorrubicina produjo un aumento en las tasas de respuesta y la supervivencia sin progresión (SSP) en relación con las de doxorrubicina sola, pero sin efecto en la supervivencia general (SG).
- El régimen de tres fármacos (doxorrubicina, cisplatino y paclitaxel) con el factor estimulante de colonias de granulocitos (G-CSF) fue significativamente superior a la combinación de cisplatino y doxorrubicina como se puede notar en lo siguiente:[Nivel de evidencia B3]
- La tasa de respuesta fue de 57 % con el régimen de tres fármacos comparada con 34 % con el régimen de cisplatino y doxorrubicina.
- La SSP fue de 8,3 meses con el régimen de tres fármacos, comparada con 5,3 meses con el régimen de cisplatino y doxorrubicina.
- La SG fue de 15,3 meses con el régimen de tres fármacos, comparada con 12,3 meses con el régimen de cisplatino y doxorrubicina.
- El régimen superior (doxorrubicina, cisplatino y paclitaxel con G-CSF) se relacionó con 12 % de neuropatía periférica de grado 3 y 27 % con la de grado 2.
Dados los efectos tóxicos y la eficacia limitada de estos regímenes, se han buscado extensamente otras opciones de tratamiento. En varios estudios de observación y estudios de fase II, se indicó actividad clínica con la combinación de quimioterapia con derivados del platino y paclitaxel para pacientes de cáncer de endometrio con enfermedad mensurable después de la cirugía primaria o la recidiva.
- El protocolo GOG-0209 (NCT000063999) del GOG, corresponde a un ensayo con ausencia de inferioridad en el que se comparó la combinación de paclitaxel, doxorrubicina y cisplatino (TAP) con G-CSF, con carboplatino y paclitaxel.
- En los resultados intermedios, actualmente disponibles en forma de sumario, se observa que la combinación de carboplatino con paclitaxel no fue inferior a la combinación TAP y conducen al uso de la combinación de carboplatino y paclitaxel como el tratamiento adyuvante estándar para la enfermedad en estadios III y IV.
- En un ensayo con pacientes de enfermedad en estadio III o IV con tumores residuales menores de 2 cm y sin compromiso de órganos parenquimatosos, se estudió el uso de cisplatino y doxorrubicina comparado con la radioterapia abdominal total.[Nivel de evidencia A1]
- Los resultados indican que el cisplatino y la doxorrubicina mejoraron la SG en comparación con la radioterapia abdominal total (ajuste de cociente de riesgos instantáneos [CRI], 0,68; intervalo de confianza [IC] 95 %, 0,52–0,89; P = 0,02; tasas de supervivencia a 5 años de 55 % del cisplatino y la doxorrubicina vs. 42 % de la radioterapia abdominal total).
- En varios ensayos se sustenta la quimioterapia combinada para pacientes de carcinosarcoma en estadio III, estadio IV y recidivante.
- En el ensayo GOG-108 de ifosfamida con cisplatino o sin este como tratamiento de primera línea para pacientes de carcinosarcomas en estadio avanzado o recidivantes, se demostró una tasa más alta de respuesta (54 vs. 34 %) y SSP más prolongada en el grupo de combinación (6 vs. 4 meses), pero no hubo una mejora significativa en la supervivencia (9 vs. 8 meses).[Nivel de evidencia A1]
- En el estudio de seguimiento GOG-0161 (NCT00003128), se utilizaron regímenes de 3 días de ifosfamida (en lugar del régimen más tóxico de 5 días utilizado en el estudio anterior) para el grupo de control e ifosfamida combinada con paclitaxel (con G-CSF desde el día 4) para el grupo de estudio.
- La combinación fue superior en términos de tasas de respuesta (45 vs. 29 %), SSP (8,4 vs. 5,8 meses) y SG (13,5 vs. 8,4 meses). El CRI para la muerte también favoreció la combinación (CRI, 0,69; IC 95 %, 0,49–0,97).[Nivel de evidencia A1]
- En este estudio, 52 % de 179 pacientes evaluables tenían enfermedad recidivante; 18 % enfermedad en estadio III y 30 % enfermedad en estadio IV. Además, hubo desequilibrios entre los grupos de tratamiento con respecto a los sitios de la enfermedad y el uso de radioterapia previa; se excluyó a 30 pacientes por características patológicas incorrectas.
- En un estudio de fase II, se evaluó el carboplatino con paclitaxel en pacientes de carcinosarcoma en estadio III, estadio IV o recidivante. Después de observarse una tasa de respuesta favorable, el GOG activó el GOG-0261 (NCT00954174), un ensayo aleatorizado de fase III de carboplatino con paclitaxel versus ifosfamida con paclitaxel. En este estudio no se reciben más pacientes y se esperan sus resultados.
Quimioterapia y radioterapia
Es posible tratar a los pacientes con enfermedad inoperable causada por un tumor que se extiende a la pared pélvica con una combinación de quimioterapia y radioterapia. El abordaje habitual de la radioterapia es el uso de una combinación de radioterapia intracavitaria y radioterapia de haz externo.
Es posible que la radioterapia sea paliativa para pacientes con recidivas localizadas (ganglios pélvicos y paraaórticos) o metástasis a distancia en sitios seleccionados. La radioterapia pélvica es a veces curativa de la recidiva vaginal pura cuando no se administró antes radioterapia.
Terapia hormonal
En los tejidos del carcinoma endometrial se suelen encontrar receptores hormonales de progesterona y estrógeno. La respuesta a la terapia hormonal se correlaciona con la presencia y las concentraciones de receptores hormonales, y el grado de diferenciación del tumor. Los pacientes con tumores con receptores de estrógeno y progesterona responden mejor al tratamiento con progestina.
Se indica la terapia hormonal cuando hay metástasis a distancia; en especial, metástasis pulmonares. Es posible que las pacientes que no son aptas para cirugía ni radioterapia se traten con fármacos progestacionales, que es el tratamiento hormonal más común. Los fármacos progestacionales producen buenas respuestas antitumorales en 15 a 30 % de las pacientes. Estas respuestas se relacionan con una mejora significativa de la supervivencia.
Los fármacos progestacionales estándar son los siguientes:
- Hidroxiprogesterona.
- Medroxiprogesterona.
- Megestrol.
Evidencia (terapia con progestina):
- En un estudio se realizó el seguimiento de 115 pacientes de cáncer de endometrio en estadio avanzado que se trataron con progestinas.
- Respondieron al tratamiento 75 % de las pacientes (42 de 56) con tumores con receptores de progesterona.
- De las pacientes sin receptores de progesterona detectables, 7 % (4 de 59) respondieron al tratamiento.
Algunas veces, la escasez de receptores predice tanto una respuesta precaria a las progestinas como una respuesta mejor a la quimioterapia citotóxica.
Se observó que otros fármacos hormonales son beneficiosos en el tratamiento del cáncer de endometrio. El tamoxifeno (20 mg dos veces al día) produce una tasa de respuesta de 20 % en pacientes que no responden a la terapia estándar con progesterona.
Si bien se evaluaron los inhibidores de la aromatasa para el tratamiento del cáncer de endometrio en estadio avanzado y recidivante, estos producen tasas más bajas de respuesta que los fármacos progestacionales.
Terapia biológica
Para el tratamiento del cáncer de endometrio se han evaluado varias sustancias biológicas.
- Inhibidores del blanco de la rapamicina en los mamíferos (mTOR).
Los cánceres de endometrio a menudo exhiben alteraciones en la vía AKT-PI3K; esto hace que los inhibidores mTOR sean una opción atractiva para el estudio clínico de pacientes con enfermedad metastásica o recidivante. En estudios de fase II del fármaco único everólimus y ridaforólimus se observó predominantemente la estabilización de la enfermedad. En un estudio de fase II de la combinación de everólimus y letrozol, se observó una tasa de respuesta de 32 %.[Nivel de evidencia C3]
- Bevacizumab.
- El bevacizumab se utilizó como fármaco único en un ensayo de fase II; la tasa general de respuesta fue de 13,5 %.[Nivel de evidencia C3]
- Bevacizumab y temsirólimus.
Opciones de tratamiento en evaluación clínica del cáncer de endometrio en estadio III, estadio IV y recidivante
Se deberá considerar a todos los pacientes con enfermedad en estadio avanzado para que participen en ensayos clínicos en los que se evalúe el tratamiento con un fármaco único o en combinación.
En los estudios de modelos de fracaso de tratamiento se encontró una tasa alta de metástasis a distancia en la parte superior del abdomen y en sitios extrabdominales. Por esta razón, es posible que los pacientes con enfermedad en estadio III sean aptos para participar en ensayos clínicos innovadores.
Las opciones de tratamiento en evaluación clínica para el cáncer de endometrio en estadio IV incluyen los siguientes fármacos:
- Paclitaxel y carboplatino con metformina o sin esta para el cáncer de endometrio en estadios III, IV y recidivante (GOG-0286B [NCT02065687]).
- Inhibidor PI3K/mTOR para el cáncer de endometrio recidivante o persistente (15-079 [NCT02549989]).
- Everólimus y letrozol, o terapia hormonal para el cáncer de endometrio recidivante o persistente (GOG-3007 [NCT02228681]).
- Everólimus, letrozol y metformina para el cáncer de endometrio avanzado o recidivante (2012-0543 [NCT01797523]).
- Paclitaxel y carboplatino versus paclitaxel e ifosfamida para pacientes de cáncer de útero, ovario, trompa de Falopio o cavidad peritoneal en estadios I a IV recién diagnosticado persistente o recidivante (GOG-0261 [NCT00954174]).
Ensayos clínicos en curso
Realizar una búsqueda avanzada en inglés de los ensayos clínicos sobre cáncer auspiciados por el NCI que ahora aceptan pacientes. La búsqueda se puede simplificar por ubicación del ensayo, tipo de tratamiento, nombre del fármaco y otros criterios. También se dispone de información general sobre los ensayos clínicos.
References
- Shih KK, Yun E, Gardner GJ, et al.: Surgical cytoreduction in stage IV endometrioid endometrial carcinoma. Gynecol Oncol 122 (3): 608-11, 2011.
- Barlin JN, Puri I, Bristow RE: Cytoreductive surgery for advanced or recurrent endometrial cancer: a meta-analysis. Gynecol Oncol 118 (1): 14-8, 2010.
- Ball HG, Blessing JA, Lentz SS, et al.: A phase II trial of paclitaxel in patients with advanced or recurrent adenocarcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 62 (2): 278-81, 1996.
- Thigpen JT, Brady MF, Homesley HD, et al.: Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 22 (19): 3902-8, 2004.
- Fleming GF, Brunetto VL, Cella D, et al.: Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 22 (11): 2159-66, 2004.
- Fleming GF, Filiaci VL, Bentley RC, et al.: Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Ann Oncol 15 (8): 1173-8, 2004.
- Arimoto T, Nakagawa S, Yasugi T, et al.: Treatment with paclitaxel plus carboplatin, alone or with irradiation, of advanced or recurrent endometrial carcinoma. Gynecol Oncol 104 (1): 32-5, 2007.
- Sovak MA, Hensley ML, Dupont J, et al.: Paclitaxel and carboplatin in the adjuvant treatment of patients with high-risk stage III and IV endometrial cancer: a retrospective study. Gynecol Oncol 103 (2): 451-7, 2006.
- Hoskins PJ, Swenerton KD, Pike JA, et al.: Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: a phase II study. J Clin Oncol 19 (20): 4048-53, 2001.
- Pectasides D, Xiros N, Papaxoinis G, et al.: Carboplatin and paclitaxel in advanced or metastatic endometrial cancer. Gynecol Oncol 109 (2): 250-4, 2008.
- Nomura H, Aoki D, Takahashi F, et al.: Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: a Japanese Gynecologic Oncology Group study (JGOG2041). Ann Oncol 22 (3): 636-42, 2011.
- Dimopoulos MA, Papadimitriou CA, Georgoulias V, et al.: Paclitaxel and cisplatin in advanced or recurrent carcinoma of the endometrium: long-term results of a phase II multicenter study. Gynecol Oncol 78 (1): 52-7, 2000.
- Miller D, Filiaci V, Fleming G, et al.: Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. [Abstract] Gynecol Oncol 125 (3): 771, 2012.
- Randall ME, Filiaci VL, Muss H, et al.: Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 24 (1): 36-44, 2006.
- Sutton G, Brunetto VL, Kilgore L, et al.: A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 79 (2): 147-53, 2000.
- Homesley HD, Filiaci V, Markman M, et al.: Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25 (5): 526-31, 2007.
- Powell MA, Filiaci VL, Rose PG, et al.: Phase II evaluation of paclitaxel and carboplatin in the treatment of carcinosarcoma of the uterus: a Gynecologic Oncology Group study. J Clin Oncol 28 (16): 2727-31, 2010.
- Wegner RE, Beriwal S, Heron DE, et al.: Definitive radiation therapy for endometrial cancer in medically inoperable elderly patients. Brachytherapy 9 (3): 260-5, 2010 Jul-Sep.
- Kupelian PA, Eifel PJ, Tornos C, et al.: Treatment of endometrial carcinoma with radiation therapy alone. Int J Radiat Oncol Biol Phys 27 (4): 817-24, 1993.
- Lentz SS: Advanced and recurrent endometrial carcinoma: hormonal therapy. Semin Oncol 21 (1): 100-6, 1994.
- Kauppila A: Oestrogen and progestin receptors as prognostic indicators in endometrial cancer. A review of the literature. Acta Oncol 28 (4): 561-6, 1989.
- Kauppila A, Friberg LG: Hormonal and cytotoxic chemotherapy for endometrial carcinoma. Steroid receptors in the selection of appropriate therapy. Acta Obstet Gynecol Scand Suppl 101: 59-64, 1981.
- Quinn MA, Campbell JJ: Tamoxifen therapy in advanced/recurrent endometrial carcinoma. Gynecol Oncol 32 (1): 1-3, 1989.
- Lindemann K, Malander S, Christensen RD, et al.: Examestane in advanced or recurrent endometrial carcinoma: a prospective phase II study by the Nordic Society of Gynecologic Oncology (NSGO). BMC Cancer 14: 68, 2014.
- Slomovitz BM, Lu KH, Johnston T, et al.: A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer 116 (23): 5415-9, 2010.
- Colombo N, McMeekin DS, Schwartz PE, et al.: Ridaforolimus as a single agent in advanced endometrial cancer: results of a single-arm, phase 2 trial. Br J Cancer 108 (5): 1021-6, 2013.
- Tsoref D, Welch S, Lau S, et al.: Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol Oncol 135 (2): 184-9, 2014.
- Slomovitz BM, Jiang Y, Yates MS, et al.: Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol 33 (8): 930-6, 2015.
- Aghajanian C, Sill MW, Darcy KM, et al.: Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol 29 (16): 2259-65, 2011.
- Alvarez EA, Brady WE, Walker JL, et al.: Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 129 (1): 22-7, 2013.
- Greven KM, Curran WJ, Whittington R, et al.: Analysis of failure patterns in stage III endometrial carcinoma and therapeutic implications. Int J Radiat Oncol Biol Phys 17 (1): 35-9, 1989.
Actualizaciones más recientes a este resumen (02/17/2023)
Los resúmenes del PDQ con información sobre el cáncer se revisan con regularidad y se actualizan a medida que se obtiene nueva información. Esta sección describe los cambios más recientes hechos a este sumario a partir de la fecha arriba indicada.
Información general sobre el cáncer de endometrio
Se actualizaron las estadísticas con el número estimado de casos nuevos y defunciones para 2023 (se citó a la American Cancer Society como referencia 1).
El Consejo editorial del PDQ sobre el tratamiento para adultos es responsable de la redacción y actualización de este resumen y mantiene independencia editorial respecto del NCI. El resumen refleja una revisión independiente de la bibliografía médica y no representa las políticas del NCI ni de los NIH. Para obtener más información sobre las políticas relativas a los resúmenes y la función de los consejos editoriales del PDQ responsables de su actualización, consultar Información sobre este resumen del PDQ e Información del PDQ® sobre el cáncer dirigida a profesionales de la salud.
Información sobre este resumen del PDQ
Propósito de este resumen
Este resumen de información del PDQ sobre el cáncer dirigido a profesionales de la salud proporciona información integral revisada por expertos y basada en la evidencia sobre el tratamiento del cáncer de endometrio. El objetivo es servir como fuente de información y ayuda para los profesionales clínicos durante la atención de pacientes. No ofrece pautas ni recomendaciones formales para tomar decisiones relacionadas con la atención sanitaria.
Revisores y actualizaciones
El Consejo editorial del PDQ sobre el tratamiento para adultos, que mantiene independencia editorial respecto del Instituto Nacional del Cáncer (NCI), revisa este resumen de manera periódica y, en caso necesario, lo actualiza. Este resumen es el resultado de una revisión bibliográfica independiente y no constituye una declaración de política del NCI ni de los Institutos Nacionales de la Salud (NIH).
Cada mes, los integrantes de este consejo revisan los artículos publicados recientemente para determinar lo siguiente:
- Si el artículo se debe analizar en una reunión del consejo.
- Si conviene añadir texto acerca del artículo.
- Si se debe reemplazar o actualizar un artículo que ya se citó.
Los cambios en los resúmenes se deciden mediante consenso de los integrantes del consejo después de evaluar la solidez de la evidencia de los artículos publicados y determinar la forma de incorporar el artículo en el resumen.
Los revisores principales del sumario sobre Tratamiento del cáncer de endometrio son:
- Fumiko Chino, MD
- Marina Stasenko, MD (New York University Medical Center)
Cualquier comentario o pregunta sobre el contenido de este resumen se debe enviar al Servicio de Información de Cáncer del Instituto Nacional del Cáncer. Por favor, no enviar preguntas o comentarios directamente a los integrantes del consejo, ya que no responderán consultas de manera individual.
Niveles de evidencia
Algunas de las referencias bibliográficas de este resumen se acompañan del nivel de evidencia. El propósito de esto es ayudar al lector a evaluar la solidez de la evidencia que respalda el uso de ciertas intervenciones o abordajes. El Consejo editorial del PDQ sobre el tratamiento para adultos emplea un sistema de jerarquización formal para asignar los niveles de evidencia científica.
Permisos para el uso de este resumen
PDQ (Physician Data Query) es una marca registrada. Se autoriza el uso del texto de los documentos del PDQ; sin embargo, no se podrá identificar como un resumen de información sobre cáncer del PDQ del NCI, salvo que el resumen se reproduzca en su totalidad y se actualice de manera periódica. Por otra parte, se permitirá que un autor escriba una oración como “En el resumen del PDQ del NCI de información sobre la prevención del cáncer de mama se describen, de manera concisa, los siguientes riesgos: [incluir fragmento del resumen]”.
Se sugiere citar la referencia bibliográfica de este resumen del PDQ de la siguiente forma:
PDQ® sobre el tratamiento para adultos. PDQ Tratamiento del cáncer de endometrio. Bethesda, MD: National Cancer Institute. Actualización:
Las imágenes en este resumen se reproducen con autorización del autor, el artista o la editorial para uso exclusivo en los resúmenes del PDQ. La utilización de las imágenes fuera del PDQ requiere la autorización del propietario, que el Instituto Nacional del Cáncer no puede otorgar. Para obtener más información sobre el uso de las ilustraciones de este resumen o de otras imágenes relacionadas con el cáncer, consultar Visuals Online, una colección de más de 2000 imágenes científicas.
Cláusula sobre el descargo de responsabilidad
Según la solidez de la evidencia, las opciones de tratamiento se clasifican como “estándar” o “en evaluación clínica”. Estas clasificaciones no se deben utilizar para justificar decisiones sobre reembolsos de seguros. Para obtener más información sobre la cobertura de seguros, consultar la página Manejo de la atención del cáncer en Cancer.gov/espanol.
Comuníquese con el Instituto Nacional del Cáncer
Para obtener más información sobre las opciones para comunicarse con el NCI, incluso la dirección de correo electrónico, el número telefónico o el chat, consultar la página del Servicio de Información de Cáncer del Instituto Nacional del Cáncer.