Instituto Nacional del Cáncer
Fecha de publicación: Feb 15, 2023
Resumen de información revisada por expertos acerca del tratamiento del cáncer epitelial de ovario, de trompas de Falopio y primario de peritoneo.
Cáncer de ovario, trompas de Falopio y primario de peritoneo
Información general sobre el cáncer epitelial de ovario, el cáncer de trompas de Falopio y el cáncer primario de peritoneo
Este sumario del PDQ aborda la estadificación y el tratamiento del cáncer epitelial de ovario, del cáncer de trompas de Falopio (CTF) y del cáncer primario de peritoneo (CPP).
Sin tener en cuenta el sitio de origen, el rasgo característico de estos cánceres es la diseminación peritoneal precoz de las metástasis. En general, se admite la inclusión del CTF y del CPP en la designación del cáncer epitelial de ovario ya que gran parte de la evidencia apuntan a una derivación común del epitelio de Müller y el tratamiento similar de estas tres neoplasias. La hipótesis de que muchos cánceres de ovario serosos de grado alto (el subtipo histológico más común) puedan surgir de lesiones precursoras que se originan en las fimbrias de las trompas de Falopio fue respaldada por hallazgos quirúrgicos de reducción de riesgo en mujeres sanas con mutaciones en BRCA1 o BRCA2. Además, los cánceres con características histológicas similares que se diagnostican como carcinomas primarios de peritoneo comparten hallazgos moleculares, como la pérdida o inactivación del gen supresor de tumores p53, y las proteínas BRCA1 o BRCA2. En consecuencia, los adenocarcinomas serosos de grado alto que surgen en las trompas de Falopio y otras partes de la cavidad peritoneal, junto con la mayoría de los cánceres epiteliales de ovario, son adenocarcinomas extrauterinos que se originan en el epitelio de Müller y se estadifican y tratan de modo similar al cáncer de ovario. Desde el año 2000, el CTF y el CPP se suelen incluir en los ensayos clínicos de cáncer de ovario.
Los cánceres de ovario de células claras y de endometrio vinculados con la endometriosis, al igual que los subtipos mucinosos, tienen diferentes firmas de expresión génica.
Los tumores de estroma y de células germinales son relativamente infrecuentes y representan menos del 10 % de los casos. Para obtener más información, consultar Tratamiento de los tumores de células germinativas del ovario y Tratamiento de los tumores de ovario de bajo potencial maligno.
Incidencia y mortalidad
El carcinoma epitelial de ovario es uno de los tumores malignos ginecológicos más comunes; casi el 50 % de los casos se presentan en mujeres mayores de 65 años. Es la quinta causa más frecuente de muerte por cáncer en las mujeres.
Número estimado de casos nuevos y defunciones por cáncer de ovario en los Estados Unidos para 2023:
- Casos nuevos: 19 710.
- Defunciones: 13 270.
Características anatómicas
Los extremos de las fimbrias de las trompas de Falopio están en estrecha aposición a los ovarios y en el espacio peritoneal, mientras que el cuerpo uterino se encuentra bajo una capa de peritoneo.
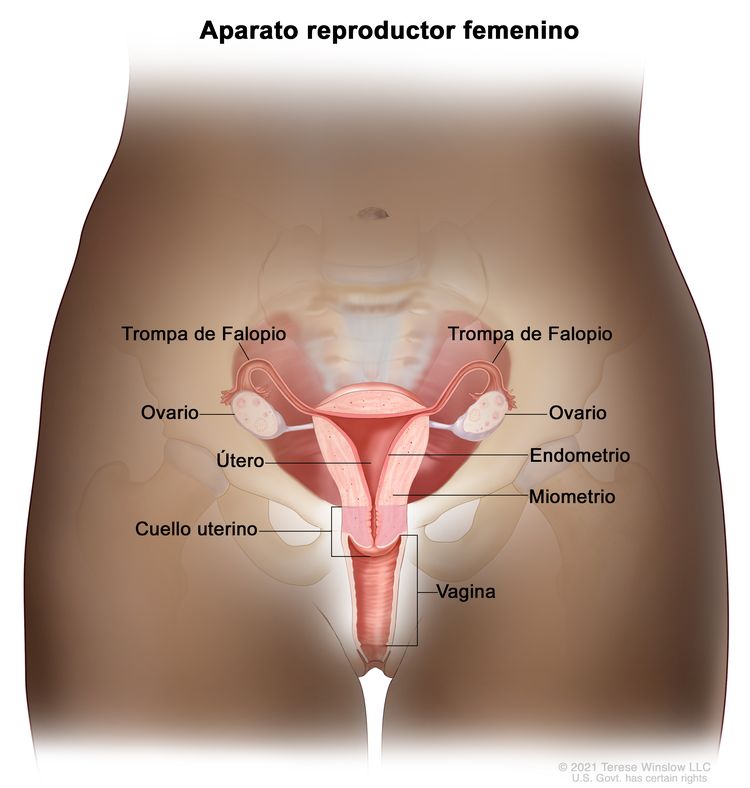 Anatomía normal del aparato reproductor femenino.
Anatomía normal del aparato reproductor femenino.
Factores de riesgo
ovarian epithelial cancerEl envejecimiento es el factor de riesgo más importante para la mayoría de los cánceres. Otros factores de riesgo del cáncer de ovario (epitelial) son los siguientes:
- Antecedentes familiares de cáncer de ovario.
- Tener un pariente de primer grado (por ejemplo, madre, hija o hermana) con la enfermedad.
- Riesgo heredado.
- Mutaciones en los genes BCRA1 o BRCA2.
- Tener otras afecciones hereditarias como cáncer colorrectal sin poliposis hereditario (CCSPH; también se llama síndrome de Lynch).
- Endometriosis.
- Terapia hormonal.
- Terapia de reemplazo hormonal posmenopáusico.
- Obesidad.
- Índice de masa corporal alto.
- Estatura alta.
Antecedentes familiares y alteraciones genéticas
El factor de riesgo más importante para el cáncer de ovario son los antecedentes de cáncer de ovario en una familiar de primer grado (madre, hija o hermana). Alrededor del 20 % de los cánceres de ovario son hereditarios y, aunque la mayoría se vincula con mutaciones en los genes BRCA1 o BRCA2, hay varios otros genes involucrados. El riesgo es más alto para las mujeres con dos o más familiares de primer grado con cáncer de ovario. El riesgo es algo menor para las mujeres con un familiar de primer grado y un familiar de segundo grado (abuela o tía) con cáncer de ovario.
En la mayoría de las familias afectadas con el síndrome de cáncer de mama y ovario, o cáncer de ovario localizado, se identificó un enlace génico al locus BRCA1 del cromosoma 17q210. El gen BRCA2, también es responsable de algunos casos de cáncer de mama y ovario heredados, y se vinculó por cartografía genética con el cromosoma 13q12.
El riesgo de por vida de padecer cáncer de ovario en pacientes portadoras de mutaciones en la línea germinal de BRCA1 aumenta de modo considerable en comparación con el riesgo de la población general. En dos estudios retrospectivos de pacientes con mutaciones en la línea germinal de BRCA1, se indica que las mujeres en estos estudios presentan mejor supervivencia que las mujeres sin mutación de BRCA1.[Nivel de evidencia C1] Es probable que la mayoría de las mujeres con la mutación de BRCA1 tengan familiares con antecedentes de cáncer de ovario o de mama; por lo tanto, es posible que las mujeres en estos estudios hayan estado más pendientes y predispuestas a participar en programas de exámenes de detección que resultaron en la detección temprana.
En el caso de mujeres con mayor riesgo, se puede considerar la ooforectomía profiláctica después de los 35 años, si ya no tendrá hijos. En un estudio de familias de 551 mujeres con mutaciones en BRCA1 o BRCA2, se encontró que de las 259 mujeres sometidas a una ooforectomía profiláctica bilateral, 2 (0,8 %) presentaron después un carcinoma papilar seroso del peritoneo y 6 (2,8 %) tenían cáncer de ovario en estadio I en el momento de la cirugía. De los 292 controles emparejados que no se sometieron a cirugía profiláctica, el 20 % presentó cáncer de ovario. La cirugía profiláctica se relacionó con una reducción del riesgo de cáncer de ovario superior al 90 % (riesgo relativo, 0,04; intervalo de confianza [IC] 95 %, 0,01–0,16), con un promedio de seguimiento de 9 años; sin embargo, es posible que los estudios de las familias tengan sesgos de selección de casos y otros factores que influyen en el cálculo del beneficio. Después de una ooforectomía profiláctica, es posible que un pequeño porcentaje de mujeres presente un carcinoma primario de peritoneo de aspecto similar al cáncer de ovario.
Cuadro clínico inicial
Es posible que el cáncer de ovario, de trompas de Falopio o de peritoneo no cause signos o síntomas tempranos. En general, cuando los signos o síntomas aparecen, el cáncer ya está en estadio avanzado. Los signos y síntomas son los siguientes:
- Dolor, inflamación o sensación de presión en el abdomen o la pelvis.
- Orinar con urgencia o frecuencia.
- Dificultad para comer o sentirse satisfecha.
- Bulto en el área de la pelvis.
- Problemas gastrointestinales como flatulencia, meteorismo o estreñimiento.
A menudo, estos síntomas no se reconocen; ello conduce a demoras en el diagnóstico. Se han realizado esfuerzos para mejorar la percepción de estos síntomas inespecíficos por parte de médicos y pacientes.
Los procedimientos de detección como la evaluación ginecológica, la ecografía vaginal y el ensayo del antígeno del cáncer 125 (CA-125) tienen un valor pronóstico bajo para detectar el cáncer de ovario en las mujeres sin factores de riesgo específicos. Como resultado de estos factores de confusión, la mortalidad anual por cáncer de ovario es cerca del 65 % de la tasa de incidencia.
La mayoría de las pacientes con cáncer de ovario tienen la enfermedad diseminada en el momento de la presentación. Es posible que la diseminación peritoneal precoz del subtipo más común de cánceres serosos de grado alto se relacione con cánceres serosos que comienzan en las fimbrias de las trompas de Falopio o en el peritoneo, lo que explica con facilidad que tales cánceres se detecten en un estadio avanzado. Por el contrario, los cánceres serosos de grado alto están poco representados entre los cánceres de ovario en estadio I. De hecho, otros tipos de cánceres de ovario están sobrerrepresentados en los cánceres detectados en los estadios I y II. Este tipo de cáncer de ovario se suele diseminar por el desprendimiento local hacia la cavidad peritoneal seguido por la implantación en el peritoneo y la invasión local del intestino y la vejiga. Se notificó que la incidencia de ganglios positivos en la primera cirugía alcanzó hasta un 24 % en pacientes con enfermedad en estadio I, un 50 % en pacientes con enfermedad en estadio II, un 74 % en pacientes con enfermedad en estadio III y un 73 % en pacientes con enfermedad en estadio IV. Los ganglios pélvicos estaban comprometidos con la misma frecuencia que los ganglios paraórticos. Las células tumorales pueden bloquear también los ganglios linfáticos del diafragma. Se cree que el consiguiente deterioro del drenaje linfático del peritoneo cumple una función en la presentación de ascitis en cáncer de ovario. Además, es común la diseminación transdiafragmática a la pleura.
Evaluación diagnóstica y estadificación
Es posible utilizar las siguientes pruebas y procedimientos para el diagnóstico y la estadificación del cáncer epitelial de ovario, cáncer de trompas de Falopio o cáncer primario de peritoneo:
- Examen físico y antecedentes.
- Examen pélvico.
- Prueba de CA-125.
- Ecografía (pélvica o transvaginal).
- Tomografía computarizada (TC).
- Tomografía por emisión de positrones (TEP).
- Imágenes por resonancia magnética (IRM).
- Radiografía de tórax.
- Biopsia.
Las concentraciones de CA-125 pueden estar elevadas en otras neoplasias malignas y problemas ginecológicos benignos, como la endometriosis. Para diagnosticar el cáncer epitelial de ovario se utilizan las concentraciones de CA-125 y las características histológicas.
Factores pronósticos
Múltiples factores afectan el pronóstico de las pacientes de cáncer de ovario. Los análisis multivariantes indican que los factores favorables más importantes son los siguientes:
- Edad más joven.
- Buen estado funcional.
- Tipos de células que no son mucinosas ni claras.
- Tumor bien diferenciado.
- Enfermedad en estadio temprano.
- Ausencia de ascitis.
- Menor volumen tumoral antes de cualquier citorreducción quirúrgica.
- Residuos tumorales más pequeños después de la cirugía citorreductora primaria.
- Portación de mutaciones en BRCA1 o BRCA2.
Para las pacientes de enfermedad en estadio I, el factor pronóstico más importante relacionado con la recaída es el grado, seguido de adherencias densas y gran volumen de ascitis. En los tumores en estadio I, hay una proporción alta de cánceres serosos de grado bajo. La derivación de estos cánceres se distingue de forma clara de los cánceres serosos de grado alto, que por lo general se presentan en los estadios III y IV. Muchos cánceres serosos de grado alto se originan en las trompas de Falopio y otras áreas extrauterinas de epitelio mülleriano.
Si el tumor es de grado 3, está muy adherido o en estadio IC, la probabilidad de recidiva y muerte por cáncer de ovario asciende hasta el 30 %.
El uso de análisis del ADN por citometría de flujo para tumores en pacientes con enfermedad en estadio I y estadio IIA, permite identificar a aquellas con riesgo alto. Las pacientes con características histológicas de células claras parecen tener el pronóstico más precario. Las pacientes con un componente importante de carcinoma de células de transición parecen tener un pronóstico mejor.
Los estudios de casos y controles indican que las pacientes portadoras de mutaciones en BRCA1 y BRCA2 reaccionan mejor a la quimioterapia, en comparación con aquellas que presentan cáncer epitelial de ovario esporádico. Esto puede ser el resultado de una deficiencia en el mecanismo de reparación del ADN homólogo en estos tumores, que aumenta la sensibilidad a los fármacos quimioterapéuticos.
Seguimiento
Debido a la baja especificidad y sensibilidad de la prueba de CA-125, el control con CA-125 seriado tal vez sea útil para pacientes sometidas a tratamiento por recidiva. No obstante, todavía no se determinó si esto ofrece un beneficio neto. Hay poca orientación acerca del seguimiento de las pacientes después de la terapia de inducción inicial. No se ha demostrado que la detección temprana por medio de imágenes ni la elevación del CA-125 alteren los resultados. Para obtener más información, consultar la sección Tratamiento del cáncer epitelial de ovario, cáncer de trompas de Falopio y cáncer primario de peritoneo recidivantes o crónicos.
References
- Levanon K, Crum C, Drapkin R: New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol 26 (32): 5284-93, 2008.
- Birrer MJ: The origin of ovarian cancer—is it getting clearer? N Engl J Med 363 (16): 1574-5, 2010.
- Dubeau L, Drapkin R: Coming into focus: the nonovarian origins of ovarian cancer. Ann Oncol 24 (Suppl 8): viii28-viii35, 2013.
- National Cancer Institute: SEER Stat Fact Sheets: Ovarian Cancer. Bethesda, Md: National Institutes of Health. Available online. Last accessed October 16, 2023.
- American Cancer Society: Cancer Facts and Figures 2023. American Cancer Society, 2023. Available online. Last accessed Dec. 15, 2023.
- Bolton KL, Ganda C, Berchuck A, et al.: Role of common genetic variants in ovarian cancer susceptibility and outcome: progress to date from the Ovarian Cancer Association Consortium (OCAC). J Intern Med 271 (4): 366-78, 2012.
- Weissman SM, Weiss SM, Newlin AC: Genetic testing by cancer site: ovary. Cancer J 18 (4): 320-7, 2012 Jul-Aug.
- Hunn J, Rodriguez GC: Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol 55 (1): 3-23, 2012.
- Pal T, Akbari MR, Sun P, et al.: Frequency of mutations in mismatch repair genes in a population-based study of women with ovarian cancer. Br J Cancer 107 (10): 1783-90, 2012.
- Gayther SA, Pharoah PD: The inherited genetics of ovarian and endometrial cancer. Curr Opin Genet Dev 20 (3): 231-8, 2010.
- Poole EM, Lin WT, Kvaskoff M, et al.: Endometriosis and risk of ovarian and endometrial cancers in a large prospective cohort of U.S. nurses. Cancer Causes Control 28 (5): 437-445, 2017.
- Pearce CL, Templeman C, Rossing MA, et al.: Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol 13 (4): 385-94, 2012.
- Mogensen JB, Kjær SK, Mellemkjær L, et al.: Endometriosis and risks for ovarian, endometrial and breast cancers: A nationwide cohort study. Gynecol Oncol 143 (1): 87-92, 2016.
- Lacey JV, Brinton LA, Leitzmann MF, et al.: Menopausal hormone therapy and ovarian cancer risk in the National Institutes of Health-AARP Diet and Health Study Cohort. J Natl Cancer Inst 98 (19): 1397-405, 2006.
- Trabert B, Wentzensen N, Yang HP, et al.: Ovarian cancer and menopausal hormone therapy in the NIH-AARP diet and health study. Br J Cancer 107 (7): 1181-7, 2012.
- Engeland A, Tretli S, Bjørge T: Height, body mass index, and ovarian cancer: a follow-up of 1.1 million Norwegian women. J Natl Cancer Inst 95 (16): 1244-8, 2003.
- Lahmann PH, Cust AE, Friedenreich CM, et al.: Anthropometric measures and epithelial ovarian cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 126 (10): 2404-15, 2010.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer: Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med 9 (4): e1001200, 2012.
- Lynch HT, Watson P, Lynch JF, et al.: Hereditary ovarian cancer. Heterogeneity in age at onset. Cancer 71 (2 Suppl): 573-81, 1993.
- Pennington KP, Swisher EM: Hereditary ovarian cancer: beyond the usual suspects. Gynecol Oncol 124 (2): 347-53, 2012.
- Piver MS, Goldberg JM, Tsukada Y, et al.: Characteristics of familial ovarian cancer: a report of the first 1,000 families in the Gilda Radner Familial Ovarian Cancer Registry. Eur J Gynaecol Oncol 17 (3): 169-76, 1996.
- Miki Y, Swensen J, Shattuck-Eidens D, et al.: A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266 (5182): 66-71, 1994.
- Easton DF, Bishop DT, Ford D, et al.: Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 52 (4): 678-701, 1993.
- Steichen-Gersdorf E, Gallion HH, Ford D, et al.: Familial site-specific ovarian cancer is linked to BRCA1 on 17q12-21. Am J Hum Genet 55 (5): 870-5, 1994.
- Wooster R, Neuhausen SL, Mangion J, et al.: Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science 265 (5181): 2088-90, 1994.
- Easton DF, Ford D, Bishop DT: Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 56 (1): 265-71, 1995.
- Struewing JP, Hartge P, Wacholder S, et al.: The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 336 (20): 1401-8, 1997.
- Rubin SC, Benjamin I, Behbakht K, et al.: Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med 335 (19): 1413-6, 1996.
- Aida H, Takakuwa K, Nagata H, et al.: Clinical features of ovarian cancer in Japanese women with germ-line mutations of BRCA1. Clin Cancer Res 4 (1): 235-40, 1998.
- Rebbeck TR, Lynch HT, Neuhausen SL, et al.: Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 346 (21): 1616-22, 2002.
- Klaren HM, van't Veer LJ, van Leeuwen FE, et al.: Potential for bias in studies on efficacy of prophylactic surgery for BRCA1 and BRCA2 mutation. J Natl Cancer Inst 95 (13): 941-7, 2003.
- Piver MS, Jishi MF, Tsukada Y, et al.: Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. A report of the Gilda Radner Familial Ovarian Cancer Registry. Cancer 71 (9): 2751-5, 1993.
- Goff BA, Mandel L, Muntz HG, et al.: Ovarian carcinoma diagnosis. Cancer 89 (10): 2068-75, 2000.
- Friedman GD, Skilling JS, Udaltsova NV, et al.: Early symptoms of ovarian cancer: a case-control study without recall bias. Fam Pract 22 (5): 548-53, 2005.
- Smith LH, Morris CR, Yasmeen S, et al.: Ovarian cancer: can we make the clinical diagnosis earlier? Cancer 104 (7): 1398-407, 2005.
- Goff BA, Mandel LS, Melancon CH, et al.: Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA 291 (22): 2705-12, 2004.
- Goff BA, Mandel LS, Drescher CW, et al.: Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer 109 (2): 221-7, 2007.
- Partridge E, Kreimer AR, Greenlee RT, et al.: Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol 113 (4): 775-82, 2009.
- van Nagell JR, Miller RW, DeSimone CP, et al.: Long-term survival of women with epithelial ovarian cancer detected by ultrasonographic screening. Obstet Gynecol 118 (6): 1212-21, 2011.
- Burghardt E, Girardi F, Lahousen M, et al.: Patterns of pelvic and paraaortic lymph node involvement in ovarian cancer. Gynecol Oncol 40 (2): 103-6, 1991.
- Berek JS, Knapp RC, Malkasian GD, et al.: CA 125 serum levels correlated with second-look operations among ovarian cancer patients. Obstet Gynecol 67 (5): 685-9, 1986.
- Atack DB, Nisker JA, Allen HH, et al.: CA 125 surveillance and second-look laparotomy in ovarian carcinoma. Am J Obstet Gynecol 154 (2): 287-9, 1986.
- Omura GA, Brady MF, Homesley HD, et al.: Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J Clin Oncol 9 (7): 1138-50, 1991.
- van Houwelingen JC, ten Bokkel Huinink WW, van der Burg ME, et al.: Predictability of the survival of patients with advanced ovarian cancer. J Clin Oncol 7 (6): 769-73, 1989.
- Neijt JP, ten Bokkel Huinink WW, van der Burg ME, et al.: Long-term survival in ovarian cancer. Mature data from The Netherlands Joint Study Group for Ovarian Cancer. Eur J Cancer 27 (11): 1367-72, 1991.
- Hoskins WJ, Bundy BN, Thigpen JT, et al.: The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol 47 (2): 159-66, 1992.
- Thigpen T, Brady MF, Omura GA, et al.: Age as a prognostic factor in ovarian carcinoma. The Gynecologic Oncology Group experience. Cancer 71 (2 Suppl): 606-14, 1993.
- Dembo AJ, Davy M, Stenwig AE, et al.: Prognostic factors in patients with stage I epithelial ovarian cancer. Obstet Gynecol 75 (2): 263-73, 1990.
- Ahmed FY, Wiltshaw E, A'Hern RP, et al.: Natural history and prognosis of untreated stage I epithelial ovarian carcinoma. J Clin Oncol 14 (11): 2968-75, 1996.
- Monga M, Carmichael JA, Shelley WE, et al.: Surgery without adjuvant chemotherapy for early epithelial ovarian carcinoma after comprehensive surgical staging. Gynecol Oncol 43 (3): 195-7, 1991.
- Kolomainen DF, A'Hern R, Coxon FY, et al.: Can patients with relapsed, previously untreated, stage I epithelial ovarian cancer be successfully treated with salvage therapy? J Clin Oncol 21 (16): 3113-8, 2003.
- Schueler JA, Cornelisse CJ, Hermans J, et al.: Prognostic factors in well-differentiated early-stage epithelial ovarian cancer. Cancer 71 (3): 787-95, 1993.
- Young RC, Walton LA, Ellenberg SS, et al.: Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials. N Engl J Med 322 (15): 1021-7, 1990.
- Gershenson DM, Silva EG, Mitchell MF, et al.: Transitional cell carcinoma of the ovary: a matched control study of advanced-stage patients treated with cisplatin-based chemotherapy. Am J Obstet Gynecol 168 (4): 1178-85; discussion 1185-7, 1993.
- Vencken PM, Kriege M, Hoogwerf D, et al.: Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol 22 (6): 1346-52, 2011.
- Safra T, Borgato L, Nicoletto MO, et al.: BRCA mutation status and determinant of outcome in women with recurrent epithelial ovarian cancer treated with pegylated liposomal doxorubicin. Mol Cancer Ther 10 (10): 2000-7, 2011.
- Rustin GJ, van der Burg ME, Griffin CL, et al.: Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet 376 (9747): 1155-63, 2010.
Clasificación celular del cáncer epitelial de ovario, de trompas de Falopio y primario de peritoneo
En el Cuadro 1 se describe la clasificación histológica del cáncer epitelial de ovario, del cáncer de trompas de Falopio (CTF) y del cáncer primario de peritoneo (CPP).
| Clasificación histológica | Subtipos histológicos |
|---|---|
| CTF = cáncer de trompas de Falopio; CPP = cáncer primario de peritoneo. | |
| Cistomas serosos | Cistoadenomas serosos benignos. |
| Cistoadenomas serosos con actividad proliferativa de las células epiteliales y anomalías nucleares, pero sin crecimiento infiltrante destructor (para obtener más información, consultar Tratamiento de tumores de ovario de bajo potencial maligno). | |
| Cistoadenocarcinomas serosos. | |
| Cistomas mucinosos | Cistomas mucinosos benignos. |
| Cistoadenomas mucinosos con actividad proliferativa de las células epiteliales y anomalías nucleares, pero sin crecimiento infiltrante destructor (neoplasia de bajo potencial maligno o neoplasia de malignidad limítrofe). | |
| Cistoadenocarcinomas mucinosos. | |
| Tumores endometrioides (similares a los adenocarcinomas en el endometrio) | Quistes endometrioides benignos. |
| Tumores endometrioides con actividad proliferativa de las células epiteliales y anomalías nucleares, pero sin crecimiento infiltrante destructor (neoplasia de bajo potencial maligno o neoplasia de malignidad limítrofe). | |
| Adenocarcinomas endometrioides. | |
| Tumores de células claras (mesonefroides) | Tumores de células claras benignos. |
| Tumores de células claras con actividad proliferativa de las células epiteliales y anomalías nucleares, pero sin crecimiento infiltrante destructor (neoplasia de bajo potencial maligno o neoplasia de malignidad limítrofe). | |
| Cistoadenocarcinomas de células claras. | |
| Tumores no clasificados que no se pueden ubicar en ninguno de los grupos anteriores | |
| Sin estudio histológico (diagnóstico con citología sola) | |
| Otros tumores malignos (los tumores malignos que no son de los tipos epiteliales comunes no se deben incluir en las categorías indicadas más arriba) | |
Información sobre los estadios del cáncer epitelial de ovario, del cáncer de trompas de Falopio y del cáncer primario de peritoneo
En ausencia de enfermedad metastásica extrabdominal, la estadificación definitiva de cáncer de ovario exige una cirugía. Todavía no se estableció la función de la cirugía para las pacientes con enfermedad en estadio IV y con enfermedad extrabdominal. Si la enfermedad parece limitarse a los ovarios o a la pelvis, es esencial que en el momento de efectuar la laparotomía se obtengan lavados peritoneales, y se examinen, y se realice una biopsia o se obtengan cepillados citológicos de los siguientes sitios:
- Diafragma.
- Ambos espacios paracólicos.
- Peritoneo pélvico.
- Ganglios paraórticos y pélvicos.
- Omento infracólico.
Estadificación de la Fédération Internationale de Gynécologie et d’Obstétrique
La Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) y el American Joint Committee on Cancer (AJCC) diseñaron la estadificación para definir el cáncer epitelial de ovario. El sistema de estadificación aprobado por FIGO para el cáncer epitelial de ovario, el cáncer de trompas de Falopio (CTF) y el cáncer primario de peritoneo (CPP) es el que más se usa.
| Estadio | Definición | Ilustración |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d’Obstétrique. | ||
| aAdaptado de FIGO Committee for Gynecologic Oncology. | ||
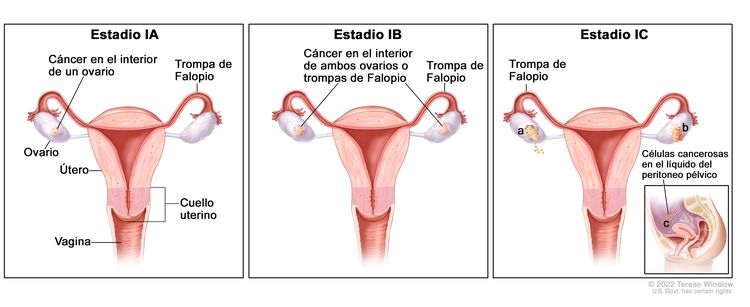
| I | Tumor confinado en los ovarios o las trompas de Falopio. |
|
| IA | Tumor limitado a un ovario (cápsula intacta) o una trompa de Falopio, no hay tumor en la superficie del ovario o la trompa de Falopio y no hay células malignas en la ascitis ni en los lavados peritoneales. | |
| IB | Tumor limitado a ambos ovarios (cápsulas intactas) o ambas trompas de Falopio, no hay tumor en la superficie del ovario o las trompas de Falopio y no hay células malignas en la ascitis o los lavados peritoneales. | |
| IC | Tumor limitado a uno o ambos ovarios, o una o ambas trompas de Falopio y se acompaña de cualquiera de las siguientes situaciones: | |
| IC1: derrame quirúrgico. | ||
| IC2: ruptura de la cápsula antes de la cirugía, o tumor en la superficie de los ovarios o las trompas de Falopio. | ||
| IC3: detección de células malignas en la ascitis o los lavados peritoneales. | ||
| Estadio | Definición | Ilustración |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d’Obstétrique. | ||
| aAdaptado del FIGO Committee for Gynecologic Oncology. | ||
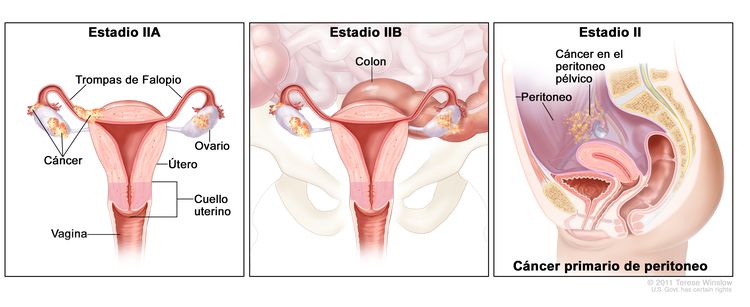
| II | Tumor que compromete uno o ambos ovarios, o una o ambas trompas de Falopio con diseminación pélvica (debajo del borde de la pelvis) o cáncer primario de peritoneo. |
|
| IIA | Diseminación o implantes en el útero, las trompas de Falopio o los ovarios. | |
| IIB | Diseminación a otros tejidos pélvicos intraperitoneales. | |
| Estadio | Definición | Ilustración |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d’Obstétrique. | ||
| aAdaptado del FIGO Committee for Gynecologic Oncology. | ||
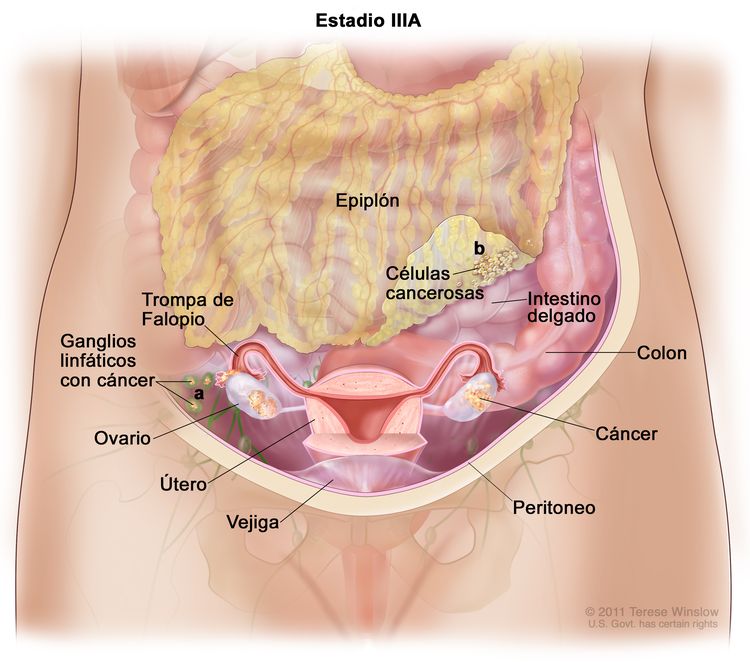
| III | Tumor que compromete uno o ambos ovarios, o una o ambas trompas de Falopio, o hay un cáncer primario de peritoneo. Además, hay diseminación al peritoneo fuera de la pelvis confirmada mediante pruebas citológicas o histológicas, o metástasis en los ganglios linfáticos retroperitoneales. | |
| IIIA1 | Compromiso solo de los ganglios linfáticos retroperitoneales (comprobados mediante pruebas citológicas o histológicas): |
|
| IIIA1(i): metástasis ≤ 10 mm en su mayor dimensión. | ||
| IIIA1(ii): metástasis >10 en su mayor dimensión. | ||
| IIIA2 | Compromiso peritoneal extrapélvico microscópico (encima del borde de la pelvis), con compromiso de ganglios linfáticos retroperitoneales o sin este. | |
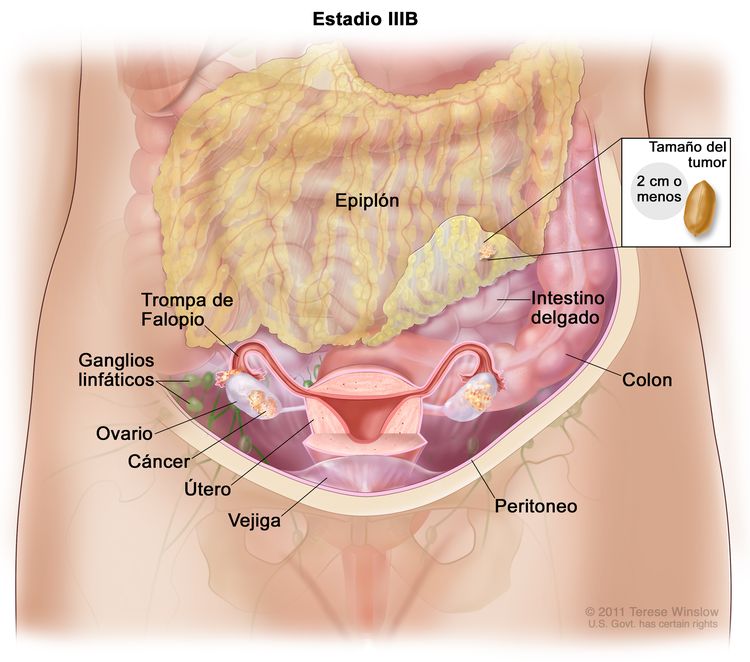
| IIIB | Metástasis peritoneales macroscópicas fuera de la pelvis de ≤2 cm en su mayor dimensión, con metástasis en los ganglios linfáticos retroperitoneales o sin estas. |
|
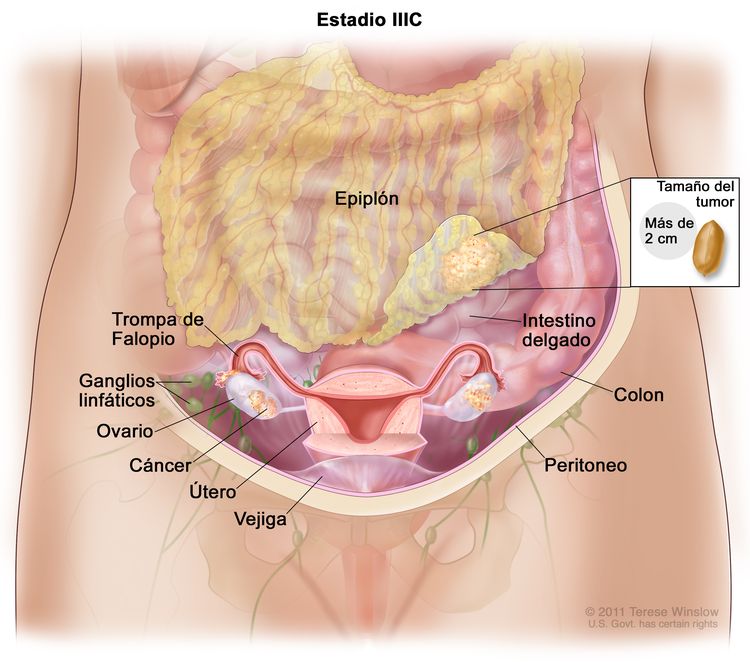
| IIIC | Metástasis peritoneales macroscópicas fuera de la pelvis de >2 cm en su mayor dimensión, con metástasis en los ganglios linfáticos retroperitoneales o sin estas (incluye extensión del tumor a la cápsula hepática y esplénica sin compromiso del parénquima en ninguno de los dos órganos). |
|
| Estadio | Definición | Ilustración |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d’Obstétrique. | ||
| aAdaptado del FIGO Committee for Gynecologic Oncology. | ||
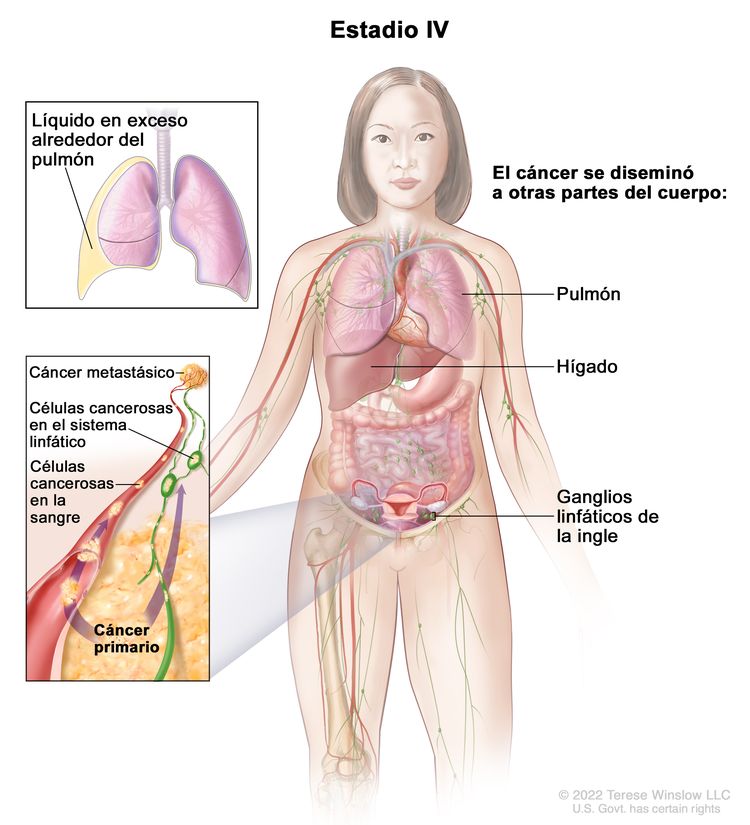
| IV | Metástasis a distancia con exclusión de metástasis peritoneales. |
|
| IVA | Derrame pleural con resultado positivo en el estudio citológico. | |
| IVB | Metástasis parenquimatosas y metástasis en los órganos extrabdominales (incluso en los ganglios linfáticos inguinales y los ganglios linfáticos fuera de la cavidad abdominal). | |
References
- Hoskins WJ: Surgical staging and cytoreductive surgery of epithelial ovarian cancer. Cancer 71 (4 Suppl): 1534-40, 1993.
- Berek JS, Renz M, Kehoe S, et al.: Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet 155 (Suppl 1): 61-85, 2021.
- Ovary, fallopian tube, and primary peritoneal carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 681-90.
Aspectos generales de las opciones de tratamiento
Las opciones de tratamiento para las pacientes de cáncer epitelial de ovario, cáncer de trompas de Falopio (CTF) y cáncer primario de peritoneo (CPP) han consistido en cirugía seguida de quimioterapia con derivados del platino.
Estadios tempranos se refiere a los estadios I y II. Sin embargo, debido a las tasas altas de recidiva en pacientes en estadio II en los ensayos de enfermedad en estadio temprano del Gynecologic Oncology Group (GOG) realizados desde 2009, los cánceres en estadio II se incluyeron en los ensayos clínicos con los de estadios más avanzados. En adelante, el estadio I permanecerá como categoría separada cuando se considere el tratamiento, pero es probable que los cánceres serosos de grado alto en estadio II se incluyan con los estadios más avanzados.
Hay numerosos ensayos clínicos en curso para refinar los tratamientos actuales y probar el valor de diferentes abordajes de los fármacos y de radioterapia posoperatoria. Las pacientes de cáncer de ovario son participantes indicadas para los ensayos clínicos. Se dispone de información sobre los ensayos clínicos en curso en el portal de Internet del NCI.
Las opciones de tratamiento para el cáncer epitelial de ovario, CTF y CPP se presentan en el Cuadro 6.
| Estadio | Opciones de tratamiento |
|---|---|
| SG = supervivencia general; PARP = poli (ADP) ribosa–polimerasa. | |
| Temprano | Cirugía con quimioterapia o sin esta |
| Avanzado | Cirugía seguida de quimioterapia con derivados del platino |
| Cirugía antes o después de la quimioterapia con derivados del platino, o terapia adicional de consolidación | |
| Cirugía antes o después de la quimioterapia con derivados del platino, y la adición de bevacizumab a la terapia de inducción o de consolidación | |
| Cirugía antes o después de la quimioterapia con derivados del platino, y la adición de inhibidores de PARP a la terapia de inducción o de consolidación | |
| Quimioterapia para pacientes que no son aptas para cirugía (aunque no se ha comprobado el efecto en la SG) | |
| Recidivante | Regímenes de quimioterapia que contienen derivados del platino |
| Bevacizumab, otros fármacos dirigidos e inhibidores de PARP, con quimioterapia o sin esta | |
| Quimioterapia | |
| Quimioterapia o bevacizumab | |
| Inhibidores de puntos de control inmunitario | |
References
- Ozols RF, Young RC: Ovarian cancer. Curr Probl Cancer 11 (2): 57-122, 1987 Mar-Apr.
- Cannistra SA: Cancer of the ovary. N Engl J Med 329 (21): 1550-9, 1993.
Tratamiento del cáncer epitelial de ovario, cáncer de trompas de Falopio y cáncer primario de peritoneo en estadio temprano
Estadios tempranos se refiere al estadio I y el estadio II. Sin embargo, debido a las tasas altas de recidiva en las pacientes en estadio II, en los ensayos de enfermedad en estadio temprano del Gynecologic Oncology Group (GOG) realizados desde 2009, los cánceres en estadio II se incluyeron en los ensayos clínicos con los de estadios más avanzados. En adelante, el estadio I permanecerá como categoría separada cuando se considere el tratamiento, pero es probable que los cánceres serosos de grado alto en estadio II se incluyan con los estadios más avanzados.
Opciones de tratamiento para el cáncer epitelial de ovario, cáncer de trompas de Falopio y cáncer primario de peritoneo
Las opciones de tratamiento para el cáncer epitelial de ovario, cáncer de trompas de Falopio (CTF) y cáncer primario de peritoneo (CPP) son las siguientes:
- Cirugía con quimioterapia o sin esta.
Cirugía con quimioterapia o sin esta
Si el tumor está bien diferenciado o moderadamente bien diferenciado, la cirugía sola puede ser el tratamiento adecuado para las pacientes con enfermedad en estadio IA y IB. La cirugía incluye histerectomía, salpingooforectomía bilateral y omentectomía. Se visualiza la superficie inferior del diafragma y se realiza una biopsia. También se llevan a cabo biopsias del peritoneo pélvico y abdominal, y biopsias de los ganglios linfáticos pélvicos y paraórticos. Los lavados peritoneales se obtienen en forma rutinaria. En el caso de pacientes que desean tener hijos y presentan tumores de grado 1, es posible que la salpingooforectomía unilateral se relacione con un riesgo bajo de recidiva.
En los Estados Unidos, excepto para el subconjunto de pacientes con diagnóstico más favorable (aquellas con enfermedad bien diferenciada en estadio IA), la evidencia obtenida en ensayos clínicos aleatorizados y controlados con enmascaramiento doble con criterios de valoración de la mortalidad total, sustentan el tratamiento adyuvante con cisplatino, carboplatino y paclitaxel.
Evidencia (cirugía con quimioterapia o sin esta):
- En dos ensayos grandes realizados en Europa, el European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm (EORTC-ACTION) y el International Collaborative Ovarian Neoplasm (MRC-ICON1 [NCT00002477]), las pacientes de carcinoma de células claras en estadios IA (grado 2) y IB (grado 3), y todas aquellas con carcinoma de células claras en estadio IC y estadio IIA, se asignaron al azar para someterse a quimioterapia adyuvante u observación.
- En el ensayo EORTC-ACTION, se exigió administrar como tratamiento por lo menos cuatro ciclos de carboplatino o quimioterapia con cisplatino. A pesar de que se supervisaron los criterios de estadificación quirúrgica, la estadificación inadecuada no se consideró un criterio de exclusión.
- La supervivencia sin recidiva (SSR) mejoró en los pacientes del grupo de quimioterapia adyuvante (cociente de riesgos instantáneos [CRI], 0,63; P = 0,02), pero la supervivencia general (SG) no resultó afectada (CRI, 0,69; intervalo de confianza [IC] 95 %, 0,44–1,08; P = 0,10).
- La SG mejoró con la quimioterapia en el subconjunto de pacientes con estadificación quirúrgica inadecuada.
- En el ensayo MRC-ICON1, se asignó al azar a las pacientes a recibir 6 ciclos de carboplatino o cisplatino como fármacos únicos, o a quimioterapia con derivados del platino (por lo general, ciclofosfamida, doxorrubicina y cisplatino) versus observación, con un criterio de participación similar al del ensayo EORTC-ACTION; sin embargo, en el ensayo MRC-ICON1 no se comprobó si hubo una estadificación quirúrgica adecuada. Cuando se combinaron los resultados de ambos ensayos, la diferencia en la SG alcanzó significación estadística.
- Tanto la SSR como la SG mejoraron de forma significativa: las tasas de supervivencia a 5 años fueron del 79 % en los pacientes que recibieron quimioterapia adyuvante versus el 70 % en aquellos que no la recibieron.
- Un análisis de los datos agrupados de ambos estudios indicaron lo siguiente:[Nivel de evidencia A1]
- Las pacientes que recibieron quimioterapia exhibieron una mejora significativa de la SSR (CRI, 0,64; IC 95 %, 0,50–0,82; P = 0,001) y la SG (CRI, 0,67; IC 95 %, 0,50–0,90; P = 0,008). La tasa de SG a 5 años fue del 82 % en las pacientes que recibieron quimioterapia y del 74 % en las pacientes que se sometieron a observación (diferencia, 8 %; IC 95 %, 2–12 %).[Nivel de evidencia A1]
- En un documento editorial adjunto se recalcó que los ensayos posteriores se deben concentrar en la identificación de pacientes que no necesiten tratamiento adicional dentro del subconjunto de pacientes de cáncer de ovario en estadio temprano. La estadificación óptima es una forma de identificar mejor a estas pacientes.
- En el ensayo EORTC-ACTION, se exigió administrar como tratamiento por lo menos cuatro ciclos de carboplatino o quimioterapia con cisplatino. A pesar de que se supervisaron los criterios de estadificación quirúrgica, la estadificación inadecuada no se consideró un criterio de exclusión.
- En el ensayo GOG-0157, se evaluó si la administración de seis ciclos de quimioterapia después de la cirugía inicial era superior a la de tres ciclos para las pacientes de cáncer epitelial de ovario de riesgo alto en estadio temprano. Las pacientes aptas fueron aquellas con enfermedad en estadio IA de grado 3 o con características histológicas de células claras, en estadio IB de grado 3 o con características histológicas de células claras, y todas las pacientes en estadio IC o en estadio II. Las pacientes se asignaron al azar para recibir 3 o 6 ciclos de la combinación de paclitaxel (175 mg/m2 administrados durante 3 horas) y carboplatino dosificado (área bajo la curva, 7,5) durante 30 minutos cada 21 días. El criterio principal de valoración fue la SSR y el estudio se diseñó con una potencia para detectar una disminución del 50 % en la tasa de recidiva a los 5 años. Se consideraron aptas a 427 pacientes en total.
- No se encontraron diferencias significativas en la incidencia acumulada de recidiva cuando se compararon 3 ciclos (25,4 %) versus 6 ciclos (20,1 %) (CRI, 0,76; IC 95 %, 0,5 – 1,13]) o la SG para 3 ciclos (81 %) versus 6 ciclos (83 %); (CRI, 1,02; P = 0,94).[Nivel de evidencia B1]
- Como se esperaba, el uso de seis ciclos se relacionó con un aumento de efectos tóxicos neurológicos de grados 3 o 4, y un aumento de efectos tóxicos hematológicos de grado 4.
- Aunque la estadificación quirúrgica fue un requisito para participar en el estudio, una auditoría descubrió que el 29 % de las pacientes no contaban con documentación completa de su cirugía o la extirpación del tumor fue insuficiente.
- En un análisis posterior de las pacientes sometidas a estadificación quirúrgica completa, tres ciclos adicionales de quimioterapia disminuyeron el riesgo de recidiva en solo un 3 %. La incidencia acumulada de recidiva a los 5 años fue del 18 % para las mujeres con enfermedad en estadio I y del 33 % para las mujeres con enfermedad en estadio II.
Dado el aumento del riesgo de recidiva en pacientes con enfermedad en estadio II y en aquellas clasificadas con un cáncer seroso de grado alto, después de 2007 el GOG optó por incluir a pacientes con enfermedad en estadio II en ensayos de cáncer de ovario en estadio avanzado (para obtener más información, consultar la sección Tratamiento del cáncer epitelial de ovario, cáncer de trompas de Falopio y cáncer primario de peritoneo en estadio avanzado). Si bien en las directrices se promulga el uso rutinario de 6 ciclos de quimioterapia, es una fuente de controversia en los análisis de subconjuntos. El GOG evaluó la quimioterapia con derivados del platino, como el paclitaxel durante 3 o 6 ciclos, en ensayos adicionales que incluyeron la administración prolongada de paclitaxel antes de eliminar gradualmente los ensayos clínicos de estadio temprano.
- En un estudio del Japanese Gynecology Oncology Group (JGOG-3016 [NCT00226915]), se inscribió a pacientes de cáncer de ovario en estadio II y se probó un cronograma de dosificación semanal versus el cronograma de dosificación convencional de primera línea cada 3 semanas para el cáncer de ovario.
Los siguientes tratamientos fueron reemplazados en gran medida por la adopción de carboplatino y paclitaxel para los primeros estadios de los cánceres de ovario de grado alto:
- Fósforo P 32 intraperitoneal o radioterapia.
- Quimioterapia sistémica con derivados del platino solos o en combinación con alquilantes.
Ensayos clínicos en curso
Realizar una búsqueda avanzada en inglés de los ensayos clínicos sobre cáncer auspiciados por el NCI que ahora aceptan pacientes. La búsqueda se puede simplificar por ubicación del ensayo, tipo de tratamiento, nombre del fármaco y otros criterios. También se dispone de información general sobre los ensayos clínicos.
References
- Young RC, Decker DG, Wharton JT, et al.: Staging laparotomy in early ovarian cancer. JAMA 250 (22): 3072-6, 1983.
- Fader AN, Java J, Ueda S, et al.: Survival in women with grade 1 serous ovarian carcinoma. Obstet Gynecol 122 (2 Pt 1): 225-32, 2013.
- Zanetta G, Chiari S, Rota S, et al.: Conservative surgery for stage I ovarian carcinoma in women of childbearing age. Br J Obstet Gynaecol 104 (9): 1030-5, 1997.
- Trimbos JB, Vergote I, Bolis G, et al.: Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J Natl Cancer Inst 95 (2): 113-25, 2003.
- Colombo N, Guthrie D, Chiari S, et al.: International Collaborative Ovarian Neoplasm trial 1: a randomized trial of adjuvant chemotherapy in women with early-stage ovarian cancer. J Natl Cancer Inst 95 (2): 125-32, 2003.
- Trimbos JB, Parmar M, Vergote I, et al.: International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst 95 (2): 105-12, 2003.
- Young RC: Early-stage ovarian cancer: to treat or not to treat. J Natl Cancer Inst 95 (2): 94-5, 2003.
- Bell J, Brady MF, Young RC, et al.: Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 102 (3): 432-9, 2006.
- Katsumata N, Yasuda M, Takahashi F, et al.: Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374 (9698): 1331-8, 2009.
- Katsumata N, Yasuda M, Isonishi S, et al.: Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 14 (10): 1020-6, 2013.
- Scambia G, Salutari V, Amadio G: Controversy in treatment of advanced ovarian cancer. Lancet Oncol 14 (10): 920-1, 2013.
- Vergote IB, Vergote-De Vos LN, Abeler VM, et al.: Randomized trial comparing cisplatin with radioactive phosphorus or whole-abdomen irradiation as adjuvant treatment of ovarian cancer. Cancer 69 (3): 741-9, 1992.
- Piver MS, Lele SB, Bakshi S, et al.: Five and ten year estimated survival and disease-free rates after intraperitoneal chromic phosphate; stage I ovarian adenocarcinoma. Am J Clin Oncol 11 (5): 515-9, 1988.
- Bolis G, Colombo N, Pecorelli S, et al.: Adjuvant treatment for early epithelial ovarian cancer: results of two randomised clinical trials comparing cisplatin to no further treatment or chromic phosphate (32P). G.I.C.O.G.: Gruppo Interregionale Collaborativo in Ginecologia Oncologica. Ann Oncol 6 (9): 887-93, 1995.
- Piver MS, Malfetano J, Baker TR, et al.: Five-year survival for stage IC or stage I grade 3 epithelial ovarian cancer treated with cisplatin-based chemotherapy. Gynecol Oncol 46 (3): 357-60, 1992.
- McGuire WP: Early ovarian cancer: treat now, later or never? Ann Oncol 6 (9): 865-6, 1995.
Tratamiento del cáncer epitelial de ovario, cáncer de trompas de Falopio y cáncer primario de peritoneo en estadio avanzado
Las opciones de tratamiento para las pacientes de cáncer epitelial de ovario, cáncer de trompas de Falopio (CTF) y cáncer primario de peritoneo (CPP) han consistido en cirugía seguida de quimioterapia con derivados del platino. Debido a las tasas altas de recidiva en pacientes en estadio II, en todos los ensayos de enfermedad en estadio temprano del Gynecologic Oncology Group (GOG) realizados desde 2009, se incluyeron los cánceres en estadio II en los ensayos clínicos con los de estadios más avanzados. En adelante, el estadio I permanecerá como categoría separada cuando se considere el tratamiento, pero es probable que los cánceres serosos de grado alto en estadio II se incluyan con los estadios más avanzados.
El enfoque más común para el cáncer de ovario en estadio avanzado es la cirugía seguida de quimioterapia adyuvante con derivados del platino. Los ensayos publicados, en los que por lo general se usa la supervivencia sin progresión (SSP) como criterio principal de valoración, se presentan en el Cuadro 7. El Gynecologic Cancer InterGroup (GCIC) aprobó la SSP como un criterio de valoración, pero posteriormente el mismo grupo lo cuestionó en una revisión sistemática y metanálisis conducida por el mismo grupo. Luego de una búsqueda en MEDLINE de ensayos clínicos aleatorizados de pacientes recién diagnosticadas con cáncer epitelial de ovario, CTF o CPP, todos los estudios con una muestra mínima de 60 pacientes publicados entre 2001 y 2016 se usaron para extraer datos individuales de SSP y supervivencia general (SG). La SSP se basó en mayor medida en las concentraciones de CA-125 confirmadas mediante examen radiológico o criterios del GCIC. De los 17 ensayos evaluados individualmente, en 5 se probó la adición de terapia de mantenimiento, en 7 se probó la adición de fármacos de inducción y en 5 se probó la terapia de intensificación. En este metanálisis no se incluyeron ensayos de inhibidores de la poli (ADP) ribosa–polimerasa. La conclusión del análisis fue que la SSP no es un sustituto indirecto de la SG, pero el análisis fue limitado por la estrecha gama de efectos del tratamiento observados y por los tratamientos posteriores al estudio.
Opciones de tratamiento para el cáncer epitelial de ovario, cáncer de trompas de Falopio y cáncer primario de peritoneo
Las opciones de tratamiento para el cáncer epitelial de ovario, cáncer de trompas de Falopio (CTF) y cáncer primario de peritoneo (CPP) en estadio avanzado son las siguientes:
- Cirugía seguida de quimioterapia con derivados del platino.
- Cirugía antes o después de la quimioterapia con derivados del platino, o terapia adicional de consolidación
- Cirugía antes o después de la quimioterapia con derivados del platino, y la adición de bevacizumab a la terapia de inducción o de consolidación.
- Cirugía antes o después de la quimioterapia con derivados del platino, y la adición de inhibidores de PARP a la terapia de inducción o de consolidación
- Quimioterapia para pacientes que no se pueden someter a cirugía (aunque no se ha comprobado el efecto en la supervivencia general [SG]).
La quimioterapia con derivados del platino es el tratamiento inicial para todas las pacientes diagnosticadas con enfermedad en estadio avanzado sometidas a resección quirúrgica y estadificadas con cáncer que se diseminó al peritoneo pélvico (estadio II) y más avanzados (estadios III y IV). La función de la cirugía para pacientes con enfermedad en estadio IV no es clara; no obstante, en la mayoría de los casos, la gran masa tumoral de la enfermedad es intrabdominal y se le aplican procedimientos quirúrgicos similares a los usados para tratar a pacientes con enfermedad en estadio II y estadio III.
Tradicionalmente, cirujanos especializados en oncología ginecológica practican la cirugía mediante laparotomía abierta con histerectomía, salpingooforectomía bilateral, omentectomía y citorreducción de implantes peritoneales (que a menudo incluye la resección del intestino o los órganos adyacentes, según sea necesario), para reducir el tumor a un tamaño microscópico, si es posible hacerse de forma segura.
En los estudios del GOG el volumen de enfermedad residual al final del procedimiento quirúrgico primario se relacionó con la supervivencia de la paciente. En una revisión bibliográfica, se observó que las pacientes con citorreducción óptima tuvieron una mediana de supervivencia de 39 meses comparada con la supervivencia de solo 17 meses de las pacientes con enfermedad residual subóptima.[Nivel de evidencia C1]
Sin embargo, en un análisis de 2655 pacientes de las 4312 pacientes inscritas en el estudio más grande del GOG (GOG-0182 [NCT00011986]), se encontró que solo la citorreducción de la enfermedad a enfermedad no visible que es R0 (es decir, resección quirúrgica completa) tuvo un efecto independiente en la supervivencia. Para obtener más información, consultar la sección Cirugía seguida por quimioterapia con derivados del platino). El GOG condujo ensayos separados para establecer la función de la terapia intraperitoneal (TI) para mujeres cuya enfermedad fue sometida a citorreducción con resultados óptimos (definida como residuo de ≤1 cm) y para aquellas cuya citorreducción fue subóptima (residuo de > 1 cm). Para obtener más información, consultar la sección Cirugía antes o después de la quimioterapia con derivados del platino, o terapia adicional de consolidación.
La citorreducción subóptima de pacientes en estadio III y estadio IV exhibe tasas inferiores de supervivencia a 5 años, pero la brecha se redujo en los ensayos en los que se incluyeron taxanos y otros fármacos a los derivados del platino. En contraste, las pacientes en estadio III tratadas con una combinación de taxano intravenoso (IV) y un derivado del platino IP además de taxano lograron una mediana de supervivencia de 66 meses en un ensayo del GOG.[Nivel de evidencia A1]
Cirugía seguida de quimioterapia con derivados del platino
Los derivados del platino, como el cisplatino o su análogo de segunda generación menos tóxico, el carboplatino, administrado solo o en combinación con otros fármacos son la base de los regímenes quimioterapéuticos que se administran. Los ensayos de varios grupos cooperativos (conducidos entre 1999 y 2010) abordaron aspectos relacionados con la intensidad óptima de la dosis para el cronograma, tanto de cisplatino como de carboplatino, y los resultados equivalentes obtenidos con cualquiera de estos fármacos derivados del platino, por lo general combinados con ciclofosfamida.
Con la introducción del taxano paclitaxel, en dos ensayos se confirmó la superioridad de cisplatino en combinación con paclitaxel, comparado con el tratamiento estándar anterior de cisplatino y ciclofosfamida. Sin embargo, en dos ensayos en los que se comparó el paclitaxel como fármaco único con cisplatino o carboplatino (ICON3 y GOG-132), no se logró confirmar tal superioridad en todos los parámetros del desenlace (es decir, respuesta, tiempo transcurrido hasta la progresión y la supervivencia) (consultar el Cuadro 7 para un listado de estos estudios).
Sobre la base de la evidencia, el tratamiento estándar inicial para pacientes de cáncer de ovario es la combinación de cisplatino o carboplatino con paclitaxel (definido como quimioterapia de inducción).
Evidencia (combinación de cisplatino o carboplatino con paclitaxel):
- Muchos consideraron que el GOG-132 demostró que el tratamiento en secuencia con cisplatino y paclitaxel equivalía a la combinación de cisplatino junto con paclitaxel; sin embargo, muchas pacientes cambiaron de grupo antes de la progresión de la enfermedad. Más aún, el grupo de solo cisplatino presentó más efectos tóxicos que el grupo de la combinación de cisplatino (75 mg/m2) y paclitaxel porque se usó una dosis de 100 mg/m2 de cisplatino por ciclo.
- En el estudio del Medical Research Council (MRC-ICON3), se comparó la monoterapia de carboplatino con la combinación de carboplatino y paclitaxel. Si bien en el estudio MRC-ICON3 hubo menos cruces tempranos de grupo que en el GOG-132, con la monoterapia de carboplatino se produjeron desenlaces similares, incluso en la SG (aunque con menos efectos tóxicos) en comparación con el tratamiento combinado.
Desde que se adoptó la combinación estándar de un derivado del platino y taxano en casi todo el mundo, los ensayos clínicos mostraron lo siguiente:
- Ausencia de inferioridad del carboplatino con paclitaxel versus cisplatino con paclitaxel.
- Ausencia de inferioridad del carboplatino con paclitaxel versus carboplatino con docetaxel.
- Ninguna ventaja, pero hubo un aumento de efectos tóxicos con la adición de epirrubicina al carboplatino además de la combinación doble con paclitaxel.
- Ausencia de inferioridad del carboplatino con paclitaxel versus la combinación doble de carboplatino en secuencia con gemcitabina o topotecán, o combinación triple con la adición de gemcitabina o doxorrubicina liposomal pegilada a la combinación doble de referencia como se muestra a continuación:
- Entre febrero de 2001 y septiembre de 2004, 4312 mujeres con cáncer epitelial de ovario, CTF o CPP en estadios III o IV que participaron en el ensayo GOG-0182 se asignaron al azar a cuatro grupos experimentales diferentes o a un tratamiento de referencia con carboplatino (área bajo la curva [ABC] 6) y paclitaxel (175 mg/m2) cada 3 semanas durante 8 ciclos. Los factores de estratificación fueron el estado de enfermedad residual y la intención de llevar a cabo una cirugía citorreductora escalonada.
- Ninguno de los regímenes experimentales fue inferior.
- Se presentaron episodios mortales atribuibles al tratamiento en menos del 1 % de las pacientes sin apego a ningún régimen.
- Con una mediana de seguimiento de 3,7 años, el riesgo relativo ajustado de muerte osciló entre 0,952 y 1,114; el grupo de control logró una SSP de 16,0 meses y una mediana de SG de 44,1 meses.
Más aún, para las pacientes en estadio III que constituían entre el 84 % y el 87 % de las pacientes, las diferencias en SSP solo se notaron cuando con la cirugía se lograron resecciones R0.
- La SSP de las pacientes con residuos mayores de 1 cm fue de 13 meses, pero la SG fue de 33 meses.
- Con residuos menores o iguales a 1 cm, la SSP fue de 16 meses y la SG fue de 40 meses.
- Con resección R0 (por ejemplo, sin residuos o con residuos microscópicos solos), la SSP fue de 29 meses y la SG fue de 68 meses.
- Entre febrero de 2001 y septiembre de 2004, 4312 mujeres con cáncer epitelial de ovario, CTF o CPP en estadios III o IV que participaron en el ensayo GOG-0182 se asignaron al azar a cuatro grupos experimentales diferentes o a un tratamiento de referencia con carboplatino (área bajo la curva [ABC] 6) y paclitaxel (175 mg/m2) cada 3 semanas durante 8 ciclos. Los factores de estratificación fueron el estado de enfermedad residual y la intención de llevar a cabo una cirugía citorreductora escalonada.
En el caso del cáncer ginecológico, a diferencia del cáncer de mama, no se exploró el paclitaxel en ensayos de fase III antes de 2004. Los resultados positivos de los ensayos del estudio Japanese Gynecologic Oncology Group (JGOG) 3016 condujeron luego a la adopción temprana de dosis divididas de paclitaxel como tratamiento estándar, pero solo con confirmación parcial de resultados superiores.
Evidencia (cronograma de tratamiento de dosis densa [semanal]):
- En un ensayo del JGOG, se inscribió a 637 pacientes (JGOG-3016 [NCT00226915]) que se asignaron al azar a 6 a 9 ciclos semanales (dosis densa) de paclitaxel (80 mg/m2) o al cronograma habitual de 180 mg/m2. Ambos regímenes se administraron con carboplatino (ABC 6) en ciclos de cada 3 semanas. El criterio principal de valoración fue la SSP con un objetivo de detectar un aumento de la SSP de 16 a 21 meses en las pacientes que recibieron el régimen a base de paclitaxel semanal. Aunque era más tóxico, el régimen semanal de paclitaxel no afectó de manera adversa la calidad de vida en comparación con el cronograma intermitente.[Nivel de evidencia B1]
Además del origen étnico, esta población del ensayo quizás se diferenció de la del GOG y otros estudios porque las pacientes eran más jóvenes (edad promedio, 57 años). De las pacientes, el 20 % tenían enfermedad en estadio II y el 33 % tenían características histológicas que no correspondían a cáncer seroso o endometrioide de grado alto. Además, el 11 % de las pacientes ingresaron en el estudio mientras estaban sometidas a tratamiento neoadyuvante, que fue una forma integral de evaluar tratamientos distintos a la quimioterapia en entornos de primera línea. Con los resultados del estudio JGOG-3016 se demostró lo siguiente:
- A los 1,5 años de seguimiento después de la suspensión del tratamiento, en las pacientes que recibieron un régimen semanal se observó una mediana de SSP de 28,0 meses (intervalo de confianza [IC] 95 %, 22,3–35,4) y en las pacientes que recibieron el régimen intermitente, la mediana de SSP fue de 17,2 meses (intervalo, 15,7–21,1; cociente de riesgos instantáneos [CRI], 0,71), lo que favoreció el régimen semanal (P = 0,0015).
- En una actualización de 2013, se descubrió un aumento de la mediana de supervivencia en las pacientes que recibieron el régimen semanal (mediana de SG, 8,3 vs. 5,1 años; P = 0,040); también cabe destacar los resultados del régimen intermitente en relación con otros ensayos clínicos de cronogramas de dosificación semanal.
- En un estudio de fase III (MITO-7 [NCT00660842]), se compararon los desenlaces de 406 pacientes asignadas a paclitaxel semanal (60 mg/m2) administrado con carboplatino semanal (ABC, 2) con los de 404 pacientes que recibieron el régimen convencional de paclitaxel y carboplatino cada 3 semanas.[Nivel de evidencia A1]
- Los resultados no permitieron confirmar la superioridad de este cronograma semanal (SSP, 18,3 meses en el grupo semanal vs. SSP, 17,3 meses en el grupo de régimen estándar [CRI, 0,96; IC 95 %, 0,80–1,16]).
- Los efectos tóxicos no difirieron. No se observó una disminución de la calidad de vida (evaluada con el cuestionario Functional Assessment of Cancer Therapy Ovarian Trial Outcome Index) en el grupo del ciclo semanal comparado con el grupo del ciclo cada 3 semanas.
- El ensayo GOG-0262 (NCT01167712) es un estudio de fase III en el que se comparó el paclitaxel semanal (80 mg/m2) a la dosis administrada cada 3 semanas (175 mg/m2), ambas acompañadas con el régimen convencional de carboplatino (ABC, 6) cada 3 semanas.[Nivel de evidencia B1] En ambos grupos se incluyó una opción de administrar bevacizumab cada 3 semanas empezando con el segundo ciclo y hasta el sexto ciclo, y seguido de bevacizumab solo durante 1 año, del mismo modo que en el GOG-0218. Esta opción se usó en cerca del 84 % de todas las pacientes.
- En general, el régimen de paclitaxel semanal no pudo prolongar la SSP comparado con el régimen administrado cada 3 semanas (14,7 vs. 14,0 meses) con un CRI para la progresión o la muerte de 0,89 (IC 95 %, 0,74–1,06).
- Sin embargo, entre las pacientes que no recibieron bevacizumab, el grupo de paclitaxel semanal tuvo una SSP significativamente prolongada (14,2 vs. 10,3 meses), con un CRI de 0,62 (IC 95 %, 0,40–0,95; P = 0,03)
- El régimen semanal de paclitaxel produjo una tasa más alta de anemia de grados 3 o 4 (36 % vs. 16 %) y de neuropatía sensorial de grados 2 a 4 (26 % vs. 18 %).
- El ensayo ICON8 (NCT01654146) es otro estudio de fase III en el que se comparó la dosis de paclitaxel semanal con la dosis administrada cada 3 semanas, en otro grupo se comparó el paclitaxel semanal con el carboplatino semanal (ABC, 2 ˣ 6 ciclos).
- En este estudio grande, no se demostraron diferencias significativas entre los grupos.
- En un estudio independiente sobre la calidad de vida, no se encontraron diferencias en la calidad de vida general entre los 3 grupos durante un análisis transversal de 9 meses, sin embargo, los grupos con cronogramas de paclitaxel semanal tuvieron una puntuación significativamente baja en los análisis longitudinales.
| Ensayo | Regímenes de tratamiento | Número de pacientes | Supervivencia sin progresión (meses) | Supervivencia general (meses) |
|---|---|---|---|---|
| ABC = área bajo la curva; Cal = calculada; EORTC = European Organisation for Research and Treatment of Cancer; GOG = Gynecologic Oncology Group; ICON = International Collaboration on Ovarian Neoplasms; JGOG = Japanese Gynecologic Oncology Group; MITO = Multicentre Italian Trials in Ovarian Cancer; MRC = Medical Research Council; SN = sin notificación. | ||||
| aLos grupos de control están en negrita. | ||||
| bResultado estadísticamente inferior (P < 0,001–< 0,05). | ||||
| cSolo citorreducción óptima. | ||||
| dCada 3 semanas durante 6 ciclos, a menos que se especifique otro esquema. | ||||
| eEn el JGOG-3016 se incluyó a pacientes en estadio II. | ||||
| fCalculada de la curva. | ||||
| GOG-111 (1990–1992)a | Paclitaxel (135 mg/m2, 24 h) y cisplatino (75 mg/m2) | 184 | 18 | 38 |
| Ciclofosfamida (750 mg/m2) y cisplatino (75 mg/m2) | 202 | 13b | 24b | |
| EORTC-55931 | Paclitaxel (175 mg/m2, 3 h) y cisplatino (75 mg/m2) | 162 | 15,5 | 35.6 |
| Ciclofosfamida (750 mg/m2) y cisplatino (75 mg/m2) | 161 | 11,5b | 25,8b | |
| GOG-132 (1992–1994) | Paclitaxel (135 mg/m2, 24 h) y cisplatino (75 mg/m2) | 201 | 14,2 | 26,6 |
| Cisplatino (100 mg/m2) | 200 | 16,4 | 30,2 | |
| Paclitaxel (200 mg/m2, 24 h) | 213 | 11,2b | 26 | |
| MRC-ICON3 | Paclitaxel (175 mg/m2, 3 h) y carboplatino (ABC, 6) | 478 | 17,3 | 36,1 |
| Carboplatino (ABC, 6) | 943 | 16,1 | 35,4 | |
| Paclitaxel (175 mg/m2, 3 h) y carboplatino (ABC, 6) | 232 | 17 | 40 | |
| Ciclofosfamida (500 mg/m2) y doxorrubicina (50 mg/m2) y cisplatino (50 mg/m2) | 421 | 17 | 40 | |
| GOG-158 (1995-1998)c | Paclitaxel (135 mg/m2, 24 h) y cisplatino (75 mg/m2)d | 425 | 14,5 | 48 |
| Paclitaxel (175 mg/m2, 3 h) y carboplatino (ABC, 6) | 415 | 15,5 | 52 | |
| JGOG-3016 (2002-2004)e | Paclitaxel (180 mg/m2) y carboplatino (ABC, 6)d | 319 | 17,5 | 62,2 |
| Paclitaxel (80 mg/m2) y carboplatino (ABC, 6) | 312 | 28,5 | 100,5 | |
| MITO-7 | Paclitaxel (175 mg/m2) y carboplatino (ABC, 6)d | 404 | 17,3 | SN |
| Paclitaxel (60 mg/m2) y carboplatino (ABC, 6) | 406 | 18,3 | SN | |
| GOG-0262 | Paclitaxel (80 mg/m2) y carboplatino (ABC, 6) con 2–6 ciclos de bevacizumab opcional, y cada 3 semanas hasta la progresión | 346 | 14.7 | Cal 42 |
| Paclitaxel (175 mg/m2) y carboplatino (ABC, 6) (× 6 ciclos) con 2–6 ciclos de bevacizumab opcional, y cada 3 semanas hasta la progresión | 346 | 14.0 | Cal 42 | |
| GOG-218 | Paclitaxel (175 mg/m2) y carboplatino (ABC, 6) (× 6 ciclos) y placebo ciclos 2–22 | 625 | 10,3 | 39,3 |
| Paclitaxel (175 mg/m2) y carboplatino (ABC, 6) (× 6 ciclos) y bevacizumab ciclos 2–6 ciclos y placebo ciclos 7–22 | 625 | 11,2 | 38,7 | |
| Paclitaxel (175 mg/m2) y carboplatino (ABC,6) (× 6 ciclos) y bevacizumab, ciclos 2–22 | 623 | 14,1 | 39,7 | |
| ICON7 | Paclitaxel (175 mg/m2) y carboplatino (ABC, 5 o 6) y bevacizumab (7,5 mg/kg) (× 6 ciclos) y bevacizumab solo, ciclos 7–18 | 764 | 19,0 | 45,5 |
| Paclitaxel (175 mg/m2) y carboplatino (ABC, 5 o 6) (× 6 ciclos) | 764 | 17,3 | 44,6 | |
| ICON8 | Paclitaxel (175 mg/m2) y carboplatino (ABC, 5 o 6) (× 6 ciclos) | 522 | 17,8 | 41f |
| Paclitaxel (80 mg/m2 semanal) y carboplatino (ABC, 5 o 4) (× 6 ciclos) | 523 | 20,8 | 41f | |
| Paclitaxel (80 mg/m2 semanal) y carboplatino (ABC, 2 semanales) (× 6 ciclos) | 521 | 21,0 | 41f | |
Cirugía antes o después de la quimioterapia con derivados del platino, o terapia adicional de consolidación
El fundamento farmacológico de administrar antineoplásicos por vía intraperitoneal (IP) se estableció a finales de la década de 1970 y a principio de la década de 1980. Cuando se estudiaron varios fármacos, en su mayor parte en el entorno de enfermedad residual mínima en el momento de la revaluación, después de que las pacientes recibieran quimioterapia inicial, el cisplatino solo o combinado fue el que recibió la mayor atención. Los desenlaces favorables con el cisplatino IP se observaron, más a menudo cuando los tumores habían respondido a la terapia con derivados del platino y cuando el volumen de los tumores era bajo (en general definidos como tumores <1 cm).
En la década de 1990, se realizaron ensayos aleatorizados para evaluar si la vía IP era superior a la vía IV. El cisplatino IP fue el denominador común de estos ensayos aleatorizados.
Evidencia (cirugía seguida de quimioterapia IP):
- El uso de cisplatino IP como parte de un abordaje inicial de pacientes con cáncer de ovario en estadio III y citorreducción óptima se sustenta, en su mayor parte, en los resultados de tres ensayos aleatorizados (SWOG-8501, GOG-0114 y GOG-0172 [NCT00003322]). En los tres estudios se probó el papel de fármacos IP (IP cisplatino en los tres ensayos y paclitaxel IP en el último ensayo) en comparación con el régimen IV estándar.
- En los tres estudios se documentó una SSP y una SG superiores en favor de la administración IP.
En particular, en el estudio más reciente, GOG-0172, se demostró lo siguiente:[Nivel de evidencia A1]
- Una mediana de supervivencia de 66 meses para pacientes en el grupo de terapia IP versus 50 meses para pacientes que recibieron cisplatino y paclitaxel IV (P = 0,03).
- Los efectos tóxicos fueron mayores en el grupo de terapia IP debido a la dosis de cisplatino por ciclo (100 mg/m22); la neuropatía sensorial se debió a la administración adicional de quimioterapia IP y por la administración sistémica de paclitaxel.
- La tasa de finalización de 6 ciclos de tratamiento también resultó más baja en el grupo de terapia IP (42 % vs. 83 %) como consecuencia de los efectos tóxicos y los problemas relacionados con el catéter.
En un análisis combinado actualizado del GOG-0114 y el GOG-0172 que incluyó a 876 pacientes con una mediana de seguimiento de 10,7 años, se notificaron los siguientes resultados:
- La mediana de supervivencia con terapia IP fue de 61,8 meses (IC 95 %, 55,5–69,5) en comparación con 51,4 meses (IC 95 %, 46,0–58,2) con terapia IV.
- La terapia IP se relacionó con una disminución del riesgo de muerte del 23 % (cociente de riesgos instantáneos [CRI] ajustado, 0,77; IC 95 %, 0,65–0,90; P = 0,002).
- La terapia IP mejoró la supervivencia de las pacientes con enfermedad residual macroscópica (≤ 1 cm) (CRI ajustado, 0,75; IC 95 %, 0,62–0,92; P = 0,006).
- El riesgo de muerte disminuyó un 12 % por cada ciclo completo de quimioterapia IP (CRI ajustado, 0,88; IC 95 %, 0,83–0,94; P< 0,001).
- Los factores relacionados con una supervivencia más precaria fueron características histológicas claras y mucinosas versus serosas (CRI ajustado, 2,79; IC 95 %, 1,83–4,24; P< 0,001), enfermedad residual macroscópica versus enfermedad no visible (CRI ajustado, 1,89; IC 95 %, 1,48–2,43; P< 0,001) y menos versus más ciclos de quimioterapia IP (CRI ajustado, 0,88; IC 95 %, 0,83–0,94; P< 0,001).
- Fue más probable que las pacientes más jóvenes completaran el régimen IP, con una disminución de las probabilidades de completarlo de un 5 % por cada año más de edad (oportunidad relativa, 0,95; IC 95 %, 0,93–0,96; P< 0,001).
- En un metanálisis patrocinado por Cochrane de todos los ensayos aleatorizados de IP versus IV, se observó un CRI de 0,79 para la supervivencia sin enfermedad y de 0,79 para la SG que favoreció a los grupos de IP.
- En otro metanálisis realizado por Cancer Care of Ontario de siete ensayos aleatorizados en los que se evaluó IP versus quimioterapia sistémica, el riesgo relativo (RR) de la progresión de la enfermedad a los 5 años según los tres ensayos que notificaron este criterio de valoración fue de 0,91 (IC 95 %, 0,85–0,98), y el RR de muerte a 5 años según los seis ensayos fue de 0,88 (IC 95 %, 0,81–0,95) para la administración IP.
- En un ensayo posterior de IP (GOG-252), se hicieron modificaciones al régimen de IP del ensayo GOG-0172 para mejorar su grado de tolerabilidad (por ejemplo, una reducción ≥25 % del total de 3 horas de cisplatino administrado; y el cambio de la administración IV menos práctica de 24 horas de paclitaxel a una administración IV de 3 horas).
En este estudio, se asignó al azar a 1560 pacientes a recibir 6 ciclos de paclitaxel IV (80 mg/m2 una vez por semana con carboplatino IV [ABC, 6] cada 3 semanas) versus paclitaxel IV (80 mg/m2 una vez por semana con carboplatino IP [ABC, 6] [el grupo de carboplatino IP]) versus paclitaxel IV una vez cada 3 semanas (135 mg/m2 durante 3 horas el día 1, 75 mg/m2 de cisplatino IP el día 2 y 60 mg/m2 de paclitaxel IP el día 8 [el grupo de cisplatino IP]). El último régimen fue el grupo de IP modificado superior del GOG-0172. Todas las participantes recibieron bevacizumab (15 mg/kg IV cada 3 semanas en los ciclos 2−22) y se agregó bevacizumab (15 mg/kg cada 3 semanas) a los tres grupos.
- La mediana de duración de la SSP fue de 24,9 meses en el grupo de carboplatino IV, de 27,4 meses en el grupo de carboplatino IP y de 26,2 meses en el grupo de cisplatino IP.
- En el subgrupo de 1380 pacientes en estadio II/III y enfermedad residual de 1 cm o menos, la mediana de SSP fue de 26,9 meses en el grupo de carboplatino IV, de 28,7 meses en el grupo de carboplatino IP y de 27,8 meses en el grupo de cisplatino IP.
- La mediana de SSP de pacientes con enfermedad en estadios II/III y sin tumor residual fue de 35,9, 38,8 y 35,5 meses, respectivamente.
- La mediana de SG de todas las pacientes inscritas fue de 75,5, 78,9 y 72,9 meses, respectivamente; la mediana de SG de las pacientes con enfermedad en estadios II/III sin tumor residual macroscópico fue de 98,8, 104,8 meses y no alcanzada, respectivamente.
- En este estudio se concluyó que, en comparación con el grupo de referencia de carboplatino IV, la SSP no aumentó significativamente con ninguno de los regímenes IP cuando se los combinó con bevacizumab.[Nivel de evidencia B1]
Cirugía antes o después de la quimioterapia con derivados del platino, y la adición de bevacizumab a la terapia de inducción o de consolidación
En dos estudios de fase III, se comparó el desenlace de la cirugía de citorreducción primaria estándar con el desenlace de la quimioterapia neoadyuvante seguida de cirugía de citorreducción de intervalo; en ambos estudios (descritos a continuación) se demostró que la SSP y la SG no fueron inferiores cuando se usó la cirugía de citorreducción primaria.
Evidencia (quimioterapia seguida de cirugía):
- Entre 1998 y 2006, en un estudio liderado por la European Organisation for the Research and Treatment of Cancer (EORTC) Gynecological Cancer Group, junto con el National Cancer Institute of Canada Clinical Trials Group (EORTC-55971 [NCT00003636]), se incluyó a 670 mujeres con cáncer epitelial de ovario, CTF y CPP en estadios lllC y lV.[Nivel de evidencia A1] Las mujeres se asignaron al azar para someterse a cirugía primaria de citorreducción seguida de por lo menos 6 ciclos de quimioterapia con derivados del platino o 3 ciclos de quimioterapia neoadyuvante con derivados del platino seguidos de cirugía de citorreducción a intervalos y, por lo menos, 3 ciclos más de quimioterapia con derivados del platino.
Los métodos incluyeron esfuerzos para asegurar la precisión del diagnóstico (por ejemplo, descartar una carcinomatosis peritoneal de origen gastrointestinal) y una estratificación por el mayor de los tumores preoperatorios (excluidos los ovarios) (<5 cm, >5 cm–10 cm, >10 cm–20 cm o >20 cm). Otros factores de estratificación fueron la institución, el método de biopsia, (es decir, laparoscopia, laparotomía o aspiración por aguja fina) y estadio tumoral (es decir, lllC o IV). El criterio principal de valoración del estudio fue la SG, con cirugía citorreductora primaria que se considera el estándar.[Nivel de evidencia A1]
- La mediana de SG con cirugía citorreductora primaria fue de 29 meses, comparada con 30 meses en las pacientes asignadas a quimioterapia neoadyuvante.
- El CRIde muerte en el grupo asignado a quimioterapia neoadyuvante seguido de citorreducción en intervalos, en comparación con los grupos asignados a cirugía de citorreducción primaria seguida de quimioterapia, fue de 0,98 (IC, 90 %, 0,84–1,13; P = 0,01 para ausencia de inferioridad).[Nivel de evidencia A1]
- La morbilidad y mortalidad posoperatorias fueron más altas en el grupo de cirugía citorreductora primaria (7,4 % con hemorragia grave y 2,5 % defunciones, en comparación con 4,1 % de hemorragia grave y 0,7 % de defunciones en el grupo neoadyuvante).
- El factor pronóstico independiente más fuerte de supervivencia prolongada fue la ausencia de tumor residual luego de la cirugía.
- El subconjunto de pacientes que exhibió una citorreducción óptima (residuo ≤1 cm) luego de una cirugía citorreductora primaria o luego de quimioterapia neoadyuvante seguida de cirugía citorreductora a intervalos, tuvo la mejor mediana de SG.
- Entre 2004 y 2010, un grupo de 87 hospitales en el Reino Unido y Nueva Zelanda inscribió a 550 mujeres con cáncer epitelial de ovario en estadio III o IV quienes fueron asignadas al azar a someterse a cirugía de citorreducción primaria seguida de 6 ciclos de quimioterapia, o 3 ciclos de quimioterapia primaria (neoadyuvante) seguida de cirugía y 3 ciclos adicionales de quimioterapia. En cambio, en el estudio EORTC, la quimioterapia consistió en carboplatino convencional (ABC, 5 o ABC, 6) y paclitaxel (175 mg/m2, en el 76 % de las pacientes), o carboplatino solo (23 % de las pacientes), o quimioterapia sin paclitaxel (1 % de las pacientes).[Nivel de evidencia A1]
Se empleó un método de minimización para asignar al azar a las pacientes en una proporción 1:1. Se estratificó a las pacientes según el centro de aleatorización, el tumor radiológicamente más grande y el régimen de quimioterapia especificados antes. El criterio principal de valoración fue la ausencia de inferioridad establecida con un límite superior unilateral del IC del 90 % para el CRIde muerte de menos de 1,18.
- Hasta mayo de 2014, habían ocurrido 451 defunciones, y el CRIde muerte favoreció la quimioterapia neoadyuvante, con un límite superior unilateral del IC del 90 % de 0,98 (IC 95 %, 0,72‒1,05).
- Los efectos adversos posoperatorios de grado 3 o 4 más frecuentes fueron la hemorragia en ambos grupos: 8 mujeres (3 %) en el grupo de cirugía de citorreducción primaria versus 14 (6 %) en el grupo de quimioterapia neoadyuvante. Se presentaron efectos tóxicos de grado 3 y 4 por la quimioterapia en 110 (49 %) de las 225 mujeres asignadas al azar a cirugía de citorreducción primaria, y en 102 (40 %) de las 253 mujeres sometidas a quimioterapia neoadyuvante; se presentó un acontecimiento mortal, sepsis neutropénica, en el grupo de quimioterapia primaria.
Estos estudios, y otros de observación y de fase III parcialmente publicados llevaron a la divulgación de una norma de práctica clínica por parte de la Society of Gynecologic Oncology y la American Society of Clinical Oncology.
La quimioterapia peritoneal hipertérmica (QIPH) es otra modalidad farmacológica para mejorar los efectos antitumorales mediante la administración directa de fármacos en las superficies intraperitoneales. Se probó inicialmente contra tumores mucinosos de origen gastrointestinal. La QIPH se está aplicando cada vez más a los cánceres de ovario, con una variación considerable en la selección de pacientes, los fármacos administrados y el tiempo hasta las temperaturas objetivo (generalmente 30 minutos a 42 °C). El papel de la QIPH sigue siendo una modalidad experimental para el tratamiento de pacientes de cáncer de ovario seroso de grado alto.
La experiencia con QIPH abarca más de dos décadas después de las publicaciones iniciales que se han resumido desde entonces. La evidencia de su uso para el cáncer de ovario incluyen un estudio aleatorizado.
- Se publicaron los resultados finales de un estudio holandés sin enmascaramiento (NCT00426257). El estudio se realizó en 8 hospitales e incluyó a 245 pacientes con diagnóstico reciente de cáncer de ovario cuya enfermedad se mantuvo por lo menos estable después de recibir 3 ciclos de carboplatino (ABC, 5–6) y 175 mg/m2 de paclitaxel , ambos administrados por vía IV cada 3 semanas. La aleatorización se realizó en el momento de la cirugía y se asignó a las pacientes a someterse a citorreducción quirúrgica sin QIPH (n = 123) o con QIPH (n = 122). A continuación, todas las pacientes recibieron 3 ciclos adicionales de quimioterapia IV. Se notificó el estudio tras una mediana de seguimiento de 4,7 años después de la cirugía. La QIPH fue perfusión de la cavidad abdominal con 100 mg/m2 de cisplatino en solución salina a 40 °C (104 °F) durante 60 minutos. Se administró tiosulfato sódico al comienzo de la perfusión con un bolo IV de 9 g/m2 en 200 ml, seguido de infusión IV continua (12 g/m2 in 1l) durante 6 horas.
- Luego de una mediana de seguimiento de 4,7 años, 209 de 245 pacientes presentaron enfermedad recidivante o murieron. En el análisis por intención de tratar, 110 de 123 pacientes del grupo de cirugía y 99 de 122 pacientes del grupo de QIPH sufrieron los eventos mencionados (CRI, 0,66; IC 95 %, 0,50–0,87; P = 0,003).
- La mediana de supervivencia sin recidiva fue 3,5 veces más larga en el grupo de QIPH (14,2 vs. 10,7 meses).
- En el grupo de cirugía, murieron 76 de 123 pacientes (62 %), pero en el grupo de QIPH habían muerto 61 de 122 pacientes (50 %) en el momento de la publicación del informe (CRI, 0,67; IC 95 %, 0,48–0,94; P = 0,02), con una mediana de SG de 33,9 meses en el grupo de cirugía y de 45,7 meses en el grupo de QIPH.
- En los dos grupos, las características de las pacientes fueron, en su mayor parte, equilibradas con respecto a la edad (mediana de edad, 61 años en ambos grupos), tipo histológico de células en pacientes de carcinoma seroso de ovario de grado alto (87 % en el grupo de cirugía y 92 % en el grupo de QIPH), enfermedad residual después de la cirugía y número de pacientes que completaron la quimioterapia adyuvante después de la cirugía (90 % en el grupo de cirugía y 94 % en el grupo de QIPH).
En las instituciones con experiencia en la administración de QIPH, los efectos adversos fueron comparables en los dos grupos. Las pacientes del grupo de QIPH tuvieron una incidencia más alta de íleo (3 % vs. 8 %), fiebre (8 % vs. 12 %) y tromboembolismo (2 % vs. 6 %) pero, en ese grupo, hubo diferencias más pequeñas en los cambios electrolíticos (5 % vs. 6 %) y neuropatías (27 % vs. 31 %) que en las pacientes del grupo de cirugía; además, ambos grupos habían recibido quimioterapia IV adicional. Es muy probable que el uso de tiosulfato sódico represente este perfil de seguridad favorable frente al cisplatino, que se administró como parte de QIPH en un ensayo de fase I publicado. La QIPH se debe considerar como opción para las pacientes que reciben terapia neoadyuvante si tienen acceso a un equipo quirúrgico con experiencia en la administración de QIPH.
En 2 ensayos de fase III (GOG-0218 [NCT00262847] e ICON7 NCT00483782]), se evaluó la función del bevacizumab como tratamiento de primera línea para el cáncer epitelial de ovario, CTF y CPP después de una citorreducción quirúrgica. En ambos ensayos se observó una mejoría modesta de la SSP cuando se añadió bevacizumab a la quimioterapia inicial y se lo continuó cada 3 semanas durante 16 y 12 ciclos adicionales como fase de mantenimiento.
Evidencia (cirugía seguida de quimioterapia y bevacizumab):
- GOG-0218, fue un ensayo aleatorizado, controlado con enmascaramiento doble que incluyó a 1873 mujeres con enfermedad en estadio lll o lV; todas recibieron quimioterapia, carboplatino (ABC, 6) y paclitaxel (175 mg/m2 durante seis ciclos). El 40 % de las mujeres tenían la enfermedad en estadio III con resección subóptima y el 26 % presentaban enfermedad en estadio IV. El criterio principal de valoración fue la SSP.[Nivel de evidencia B1] Las participantes se asignaron al azar para recibir lo siguiente:
- Quimioterapia y placebo (ciclos 2–22) (grupo de control).
- Quimioterapia y bevacizumab (15 mg/kg ciclos 2–6), seguido de placebo (ciclos 7–22) (grupo con bevacizumab inicial).
- Quimioterapia más bevacizumab (15 mg/kg ciclos 2–22) (grupo de bevacizumab durante todo el ensayo).
Los resultados del ensayo demostraron lo siguiente:
- No hubo diferencia en SSP entre el grupo de control y el grupo de bevacizumab inicial.
- Hubo un aumento con significación estadística en la SSP en el grupo de bevacizumab durante todo el ensayo cuando se la comparó con la del grupo de control (14,1 vs. 10,3 meses), con un CRI de progresión de la enfermedad o muerte de 0,717 en el grupo de bevacizumab durante todo el ensayo (IC 95 %, 0,625–0,824; P< 0,001).
- La mediana de SG fue 39,3 meses en el grupo de control, de 38,7 meses en el grupo de bevacizumab inicial y de 39,7 meses en el grupo de bevacizumab durante todo el ensayo.
- No hubo diferencia en la calidad de vida entre los tres grupos. La hipertensión de grado 2 o más alto fue más común en el grupo de bevacizumab que en el de placebo.
- Hubo más defunciones relacionadas con el tratamiento en el grupo de bevacizumab durante todo el ensayo (10 de 607, 2,3 %) que en el grupo de control (6 de 601, 1,0 %).
- En el ICON 7, se asignó al azar a 1528 mujeres luego de la cirugía inicial a recibir quimioterapia —carboplatino (ABC, 5 o 6) más paclitaxel (175 mg/m2 durante 6 ciclos)— o a quimioterapia más bevacizumab (7,5 mg/kg durante 6 ciclos), seguido de bevacizumab solo durante 12 ciclos adicionales. El 9 % de las pacientes presentaban tumores de grado alto en estadio temprano; el 70 % presentaban enfermedad en estadios lllC o lV, y el 26 % presentaban más de 1 cm de tumor residual antes de iniciar la quimioterapia. La SSP fue la medida principal del desenlace.[Nivel de evidencia B1]
- La mediana de SSP fue de 17,3 meses en el grupo de control y de 19 meses en el grupo de bevacizumab. El CRI de progresión de la enfermedad o el CRI de muerte en el grupo de bevacizumab fue de 0,81 (IC 95 %, 0,70–0,94; P = 0,004).
- Los efectos adversos de grado 3 o más alto fueron más comunes en el grupo de bevacizumab, con aumento de hemorragia, hipertensión (de grado 2 o más alto), episodios tromboembólicos (grado 3 o más alto) y perforaciones gastrointestinales.
- La calidad de vida no difirió entre los dos grupos.
- En 2015, los autores de ICON7 notificaron un análisis actualizado de la supervivencia.
- No hubo diferencias significativas con 44,6 meses (IC 95 %, 43,2–45,9) en las pacientes que recibieron quimioterapia estándar versus 45,5 meses (44,2–46,7) en las pacientes que recibieron bevacizumab con la quimioterapia de inducción y que luego completaron 1 año de mantenimiento con bevacizumab (P de rango logarítmico = 0,85).
Estos dos estudios respaldaron la aprobación del bevacizumab por la Administración de Alimentos y Medicamentos de los Estados Unidos (FDA) en el entorno de primera línea, tanto durante la terapia de inducción como en la terapia de consolidación. Bevacizumab obtuvo la aprobación por primera vez en el entorno de pacientes resistentes a los derivados del platino (ensayo AURELIA [NCT00976911]).
Cirugía antes o después de la quimioterapia con derivados del platino, y la adición de inhibidores de la poli (ADP) ribosa–polimerasa a la terapia de inducción o de consolidación
La poli (ADP) ribosa–polimerasa (PARP) es una familia de enzimas que participan en la reparación por escisión de bases de las roturas monocatenarias del ADN. En pacientes con deficiencia en la recombinación homóloga, incluso pacientes con mutaciones en la línea germinal de BRCA1 o BRCA2 (gBRCA) o con tumores que exhiben deficiencia en la recombinación homóloga fuera de la línea germinal, la inhibición de PARP produce roturas bicatenarias del ADN. Los mecanismos humanos de reparación del ADN descansan en su mayoría en una copia intacta del gen. Las células con roturas bicatenarias suelen ser el objetivo de la muerte celular. Esta susceptibilidad del BRCA deficiente o el BRCA mutante en las células para la inhibición de PARP estimuló el desarrollo clínico de esta clase de sustancias. Al principio, estas sustancias se probaron en mujeres que se habían tratado antes con quimioterapia. Para obtener más información, consultar la sección Bevacizumab, otros fármacos dirigidos e inhibidores de poli (ADP-ribosa)–polimerasa (PARP), con quimioterapia o sin esta.
Evidencia (cirugía antes o después de la quimioterapia e inhibidores de PARP):
- En un ensayo de fase III con enmascaramiento doble (SOLO-1) (NCT01844986), se comparó el mantenimiento de olaparib (comprimidos de 300 mg, 2 veces por día) con un placebo. Las pacientes inscritas con un diagnóstico reciente de cáncer de ovario seroso o endometrioide en estadio avanzado con mutaciones en BRCA1 o BRCA2, exhibieron una respuesta clínica completa o parcial después de recibir quimioterapia con derivados del platino. El estudio de 391 pacientes asignadas al azar se extendió entre septiembre de 2013 y marzo de 2015. De esas pacientes, 260 se asignaron para recibir olaparib y 131 pacientes para recibir un placebo. Todas las pacientes, excepto 3, tenían mutaciones de la línea germinal en BRCA1 (n = 191) o BRCA2 (n = 66). El análisis del criterio principal de valoración se interrumpió después de 2 años si no había indicios de enfermedad o se continuó hasta que la evaluación del investigador indicó la progresión de la enfermedad. Se permitió que las pacientes con respuesta parcial a los 2 años se sometieran a la intervención de modo enmascarado. Aunque no se especificó el cruce de las pacientes entre los grupos, las pacientes podían recibir tratamiento después de la interrupción a juicio del investigador. El criterio principal de valoración fue la SSP, que se definió como el período desde la aleatorización hasta la progresión objetiva de la enfermedad captada en pruebas de imágenes (cada 12 semanas durante un máximo de 3 años) o hasta la muerte por cualquier causa.
- Al cabo de una mediana de seguimiento de 41 meses, el riesgo de progresión de la enfermedad o de muerte fue un 70 % más bajo con olaparib que con un placebo (cálculos de Kaplan-Meier de la SSP a los 3 años, 60 % vs. 27 %; CRI, 0,3; IC 95 %, 0,23–0,41; P< 0,001).
- Se presentaron efectos adversos de grados 3 y 4 en el 39 % de las pacientes del grupo que recibió olaparib versus el 18 % de aquellas que recibieron un placebo. Los episodios más comunes en el grupo de olaparib fueron fatiga, vómitos y anemia. Se interrumpió la administración del fármaco para el 12 % de las pacientes que recibieron olaparib versus el 2 % de quienes recibieron un placebo.
- No se presentaron cambios significativos en la calidad de vida en ninguno de los grupos.[Nivel de evidencia B1]
- En un análisis actualizado, los resultados indicaron una reducción en el riesgo de progresión de la enfermedad o muerte de la siguiente manera:
- Reducción del 69 % con olaparib (CRI, 0,31; IC 95 %, 0,21−0,46) en comparación con el 63 % de reducción con un placebo (CRI, 0,37; IC 95 %, 0,24–0,58) en las pacientes sometidas a cirugía inicial o cirugía de intervalo.
- Reducción del 56 % con olaparib (CRI, 0,44; IC 95 %, 0,25−0,77) en comparación con el 67 % de reducción con un placebo (CRI, 0,33; IC 95 %, 0,23−0,46) en las pacientes con enfermedad residual o sin enfermedad residual después de la cirugía.
- Reducción del 66 % con olaparib (CRI, 0,34; IC 95 %, 0,24−0,47) en comparación con el 69 % de reducción con un placebo (CRI, 0,31; IC 95 %, 0,18−0,52) en las mujeres con una respuesta clínica completa o respuesta parcial al inicio.
- Reducción del 59 % con olaparib (CRI, 0,41; IC 95 %, 0,30−0,56) en comparación con el 80 % de reducción con un placebo (CRI, 0,20; IC 95 %, 0,10−0,37) en las pacientes con una mutación en BRCA1 o BRCA2.
- En un ensayo de fase III con enmascaramiento doble (PRIMA [NCT02655016]), se comparó el mantenimiento con niraparib (comprimidos de 300 mg una vez por día y luego modificado a 200 mg para mujeres de <77 kg o con un recuento inicial de plaquetas de <150 000/µl) versus placebo en pacientes con cáncer de ovario seroso de grado alto antes del último ciclo de quimioterapia con derivados del platino. Se presentó deficiencia en la recombinación homóloga, determinada por myChoice (Myriad), en el 50,9 % de las pacientes. En una aleatorización 2:1 (niraparib, n = 487; placebo, n = 246) entre junio de 2016 y mayo de 2018, las pacientes se asignaron para una comparación del criterio principal de valoración de la SSP según su deficiencia en la recombinación homóloga (50,9 %) y para la población general.
- Al cabo de una mediana de seguimiento de 13,8 meses, el riesgo de progresión en la población con deficiencia en la recombinación homóloga exhibió un CRI de 0,43 (IC 95 %, 0,31−0,59; P< 0,001), que correspondió a una mediana de SSP de 21,9 meses versus 10,4 meses, lo que fue favorable para el grupo del fármaco comparado con el grupo de placebo. En la población general seleccionada para este ensayo, el CRI fue de 0,62, que correspondió a una mediana de SSP de 13,8 meses versus 8,2 meses (IC 95 %, 0,50−0,76; P< 0,001).
- Los efectos adversos de grado 3 o más altos (no mortales) fueron anemia en el 31 % de las pacientes, trombocitopenia en el 28,7 % de las pacientes y neutropenia en el 12,8 % de las pacientes; 58 de las 307 interrupciones del niraparib se debieron a efectos adversos versus 5 de las 175 interrupciones del placebo.
- En el estudio de fase III controlado con placebo VELIA/GOG-3005 (NCT02470585) , se evaluó la eficacia del veliparib oral que se añadió a la quimioterapia de inducción de primera línea con carboplatino/paclitaxel y que se continuó como quimioterapia de mantenimiento. En el estudio, se asignó al azar a 1140 pacientes en una proporción 1:1:1 para recibir quimioterapia y placebo seguidos de mantenimiento con placebo; quimioterapia y veliparib seguidos de mantenimiento con placebo; y quimioterapia acompañada de inducción y mantenimiento con veliparib (se llamó "veliparib durante todo el tratamiento"). Las dosis de veliparib fueron de 150 mg 2 veces por día durante la inducción, y las pacientes que completaron 6 ciclos sin progresión, recibieron veliparib en monoterapia (o un placebo idéntico) en dosis de 300 mg 2 veces por día durante 2 semanas (se llamó "período de transición") y, si no se observaban efectos secundarios limitantes de la dosis entonces la dosis se aumentó a 400 mg 2 veces por día durante 30 ciclos adicionales de 3 semanas del fármaco oral. En el estudio se inscribieron pacientes desde julio de 2015 hasta julio de 2017; los datos se analizaron al cabo de una mediana de duración del seguimiento de 28 meses. Del mismo modo que en el estudio PRIMA mencionado antes, los análisis de eficacia se realizaron en tres poblaciones inclusivas secuenciales: 1) la cohorte con la mutación en BRCA; 2) la cohorte con deficiencia en la recombinación homóloga (que incluyó la cohorte anterior), y 3) la población por intención de tratar.
- Al cabo de una mediana de seguimiento de 28 meses, la cohorte con la mutación en BRCA exhibió una SSP de 34,7 meses cuando recibió veliparib durante todo el tratamiento versus 22,0 meses en el grupo de quimioterapia sola (no se comparó la adición de inducción con veliparib sin mantenimiento con veliparib). Estos valores correspondieron a un CRI de 0,44 (IC 95 %, 0,28−0,68; P< 0,001).
- Para la población con deficiencia en la recombinación homóloga, la SSP se presentó al cabo de una mediana de 31,9 meses en el grupo que recibió veliparib durante todo el tratamiento versus 20,5 meses en el grupo de quimioterapia sola, con un CRI de 0,57 (IC 95 %, 0,43−0,76; P< 0,001).
- En la población general seleccionada, la mediana de SSP fue de 23,5 meses en el grupo que recibió veliparib durante todo el tratamiento versus 17,3 meses en el grupo de quimioterapia sola, con un CRI de 0,68 (IC 95 %, 0,56−0,83; P< 0,001).
- El veliparib contribuyó a una tasa más alta de anemia y trombocitopenia cuando se combinó con quimioterapia, y contribuyó en general a náuseas y fatiga. Los efectos adversos que no se relacionaron con la progresión durante el mantenimiento condujeron a la interrupción del fármaco en 82 pacientes. En la cohorte de veliparib durante todo el tratamiento, 40 de las 377 pacientes que iban a iniciar terapia de mantenimiento con veliparib retiraron el consentimiento para recibir el fármaco del ensayo; en la cohorte de quimioterapia con placebo, 22 de las 371 pacientes retiraron el consentimiento para recibir el fármaco del ensayo; y en el grupo de veliparib solo como mantenimiento, 24 de las 383 pacientes retiraron el consentimiento para recibir el fármaco del ensayo (grupo que no se analizó más en las comparaciones).
- En el ensayo controlado con placebo PAOLA1 (NCT02477644), se comparó la quimioterapia de primera línea con carboplatino/paclitaxel seguida por bevacizumab de mantenimiento durante 2 años, con la inclusión de olaparib versus placebo en la fase de mantenimiento. En este estudio participaron 537 pacientes que se evaluaron para detectar mutaciones en BRCA (incluso mutaciones somáticas) y una cohorte con deficiencia de recombinación no homóloga.
- La SSP en las pacientes con mutaciones en BRCA (29 %) fue de 37,2 meses en el grupo de bevacizumab y olaparib versus 21,7 meses en el grupo de mantenimiento con bevacizumab solo, lo que correspondió a un CRI de 0,31.
- En la población sin mutaciones en BRCA, el CRI fue de 0,71, lo que correspondió a una mediana de SSP de 18,9 meses versus 16,0 meses.
- En la población general, el CRI fue de 0,59, lo que correspondió a una SSP de 22,1 meses para el grupo de bevacizumab y olaparib versus 16,6 meses para el grupo de mantenimiento con bevacizumab solo.
Otros ensayos de terapia de consolidación o mantenimiento
Se realizaron ensayos de fase III de terapia de consolidación o mantenimiento con fármacos citotóxicos, moléculas pequeñas, vacunas y radioinmunoconjugados con resultados negativos. La extensión de la administración de paclitaxel produjo una prolongación modesta de la SSP en ensayos aleatorizados, sin embargo, no se adoptó como un tratamiento estándar después de un ensayo subsecuente.
Evidencia (otras terapias de consolidación o mantenimiento):
- El estudio JAVELIN OVARIAN 100 (NCT02718417) fue el primer ensayo aleatorizado publicado de un inhibidor de puntos de control (avelumab, un anticuerpo anti-ligando de muerte programada 1 [PD-L1]) en pacientes con cáncer epitelial de ovario, cáncer de trompas de Falopio o cáncer de peritoneo sin tratamiento previo. Entre mayo de 2016 y enero de 2018, se asignaron al azar a 998 pacientes a recibir quimioterapia con inducción de avelumab seguida por mantenimiento con avelumab (n = 331); quimioterapia seguida por mantenimiento con avelumab (n = 332) o quimioterapia seguida por observación (n = 335).
- El estudio se terminó durante el análisis interino debido a la imposibilidad de alcanzar el criterio principal de valoración de mejora en la SSP.
- Más pacientes en los grupos de avelumab suspendieron el tratamiento del estudio y presentaron eventos adversos graves de grados 3 a 5 que los pacientes en el grupo de quimioterapia sola.
- En el estudio multicéntrico aleatorizado de enmascaramiento doble controlado con placebo de fase III, IMagyn050 (NCT03038100), se estudió la quimioterapia con derivados del platino y el bevacizumab con atezolizumab, un anticuerpo anti–PD-L1, o sin este. En el estudio, se notificaron resultados negativos para los grupos de pacientes en tratamiento de primera línea que recibieron atezolizumab.
Opciones de tratamiento en evaluación clínica
Están en curso ensayos con fármacos antiangiogénicos (distintos del bevacizumab) e inhibidores de PARP. PARP es una familia de enzimas que participan en la reparación por escisión de bases de las roturas monocatenarias del ADN. En pacientes con deficiencia en la recombinación homóloga, incluso pacientes con mutaciones en la línea germinal de BRCA1 o BRCA2 (gBRCA) o con tumores que exhiben deficiencia en la recombinación homóloga fuera de la línea germinal, la inhibición de PARP produce roturas bicatenarias del ADN. Los mecanismos humanos de reparación del ADN descansan en su mayoría en una copia intacta del gen; las células con roturas bicatenarias suelen ser el objetivo de la muerte celular. Esta susceptibilidad a la inhibición de PARP de las células con deficiencia del BRCA o una mutación en BRCA ha estimulado el desarrollo clínico de esta clase de fármacos. Debido a que una característica de la deficiencia en la recombinación homóloga es la sensibilidad a los compuestos de platino, se espera que una población de pacientes sensibles a los derivados del platino exhiba mayor deficiencia en la recombinación homóloga, y es muy probable que se beneficie de la inhibición de PARP.
Para obtener más información sobre ensayos clínicos en curso, consultar el portal de Internet del NCI.
Ensayos clínicos en curso
Realizar una búsqueda avanzada en inglés de los ensayos clínicos sobre cáncer auspiciados por el NCI que ahora aceptan pacientes. La búsqueda se puede simplificar por ubicación del ensayo, tipo de tratamiento, nombre del fármaco y otros criterios. También se dispone de información general sobre los ensayos clínicos.
References
- Paoletti X, Lewsley LA, Daniele G, et al.: Assessment of Progression-Free Survival as a Surrogate End Point of Overall Survival in First-Line Treatment of Ovarian Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open 3 (1): e1918939, 2020.
- Hoskins WJ: Surgical staging and cytoreductive surgery of epithelial ovarian cancer. Cancer 71 (4 Suppl): 1534-40, 1993.
- Hoskins WJ, Bundy BN, Thigpen JT, et al.: The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol 47 (2): 159-66, 1992.
- Hoskins WJ, McGuire WP, Brady MF, et al.: The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol 170 (4): 974-9; discussion 979-80, 1994.
- Bristow RE, Tomacruz RS, Armstrong DK, et al.: Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 20 (5): 1248-59, 2002.
- Horowitz NS, Miller A, Rungruang B, et al.: Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol 33 (8): 937-43, 2015.
- Omura GA, Brady MF, Homesley HD, et al.: Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J Clin Oncol 9 (7): 1138-50, 1991.
- Armstrong DK, Bundy B, Wenzel L, et al.: Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354 (1): 34-43, 2006.
- Markman M, Reichman B, Hakes T, et al.: Impact on survival of surgically defined favorable responses to salvage intraperitoneal chemotherapy in small-volume residual ovarian cancer. J Clin Oncol 10 (9): 1479-84, 1992.
- Markman M: Intraperitoneal chemotherapy. Semin Oncol 18 (3): 248-54, 1991.
- Levin L, Simon R, Hryniuk W: Importance of multiagent chemotherapy regimens in ovarian carcinoma: dose intensity analysis. J Natl Cancer Inst 85 (21): 1732-42, 1993.
- McGuire WP, Hoskins WJ, Brady MF, et al.: Assessment of dose-intensive therapy in suboptimally debulked ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 13 (7): 1589-99, 1995.
- Bolis G, Favalli G, Danese S, et al.: Weekly cisplatin given for 2 months versus cisplatin plus cyclophosphamide given for 5 months after cytoreductive surgery for advanced ovarian cancer. J Clin Oncol 15 (5): 1938-44, 1997.
- Alberts DS, Green S, Hannigan EV, et al.: Improved therapeutic index of carboplatin plus cyclophosphamide versus cisplatin plus cyclophosphamide: final report by the Southwest Oncology Group of a phase III randomized trial in stages III and IV ovarian cancer. J Clin Oncol 10 (5): 706-17, 1992.
- du Bois A, Lück HJ, Meier W, et al.: A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 95 (17): 1320-9, 2003.
- Neijt JP, Engelholm SA, Tuxen MK, et al.: Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol 18 (17): 3084-92, 2000.
- Muggia FM, Braly PS, Brady MF, et al.: Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol 18 (1): 106-15, 2000.
- The International Collaborative Ovarian Neoplasm Group: Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet 360 (9332): 505-15, 2002.
- Ozols RF, Bundy BN, Greer BE, et al.: Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 21 (17): 3194-200, 2003.
- Vasey PA, Jayson GC, Gordon A, et al.: Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst 96 (22): 1682-91, 2004.
- Kristensen GB, Vergote I, Stuart G, et al.: First-line treatment of ovarian cancer FIGO stages IIb-IV with paclitaxel/epirubicin/carboplatin versus paclitaxel/carboplatin. Int J Gynecol Cancer 13 (Suppl 2): 172-7, 2003 Nov-Dec.
- Bookman MA, Brady MF, McGuire WP, et al.: Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol 27 (9): 1419-25, 2009.
- Hoskins PJ: Triple cytotoxic therapy for advanced ovarian cancer: a failed application, not a failed strategy. J Clin Oncol 27 (9): 1355-8, 2009.
- Katsumata N, Yasuda M, Takahashi F, et al.: Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374 (9698): 1331-8, 2009.
- Katsumata N, Yasuda M, Isonishi S, et al.: Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 14 (10): 1020-6, 2013.
- Harano K, Terauchi F, Katsumata N, et al.: Quality-of-life outcomes from a randomized phase III trial of dose-dense weekly paclitaxel and carboplatin compared with conventional paclitaxel and carboplatin as a first-line treatment for stage II-IV ovarian cancer: Japanese Gynecologic Oncology Group Trial (JGOG3016). Ann Oncol 25 (1): 251-7, 2014.
- Pignata S, Scambia G, Katsaros D, et al.: Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 15 (4): 396-405, 2014.
- Chan JK, Brady MF, Penson RT, et al.: Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N Engl J Med 374 (8): 738-48, 2016.
- Clamp AR, James EC, McNeish IA, et al.: Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 394 (10214): 2084-2095, 2019.
- Blagden SP, Cook AD, Poole C, et al.: Weekly platinum-based chemotherapy versus 3-weekly platinum-based chemotherapy for newly diagnosed ovarian cancer (ICON8): quality-of-life results of a phase 3, randomised, controlled trial. Lancet Oncol 21 (7): 969-977, 2020.
- McGuire WP, Hoskins WJ, Brady MF, et al.: Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 334 (1): 1-6, 1996.
- Mahner S, Burges A: Quality of life as a primary endpoint in ovarian cancer trials. Lancet Oncol 15 (4): 363-4, 2014.
- Oza AM, Cook AD, Pfisterer J, et al.: Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol 16 (8): 928-36, 2015.
- Howell SB, Zimm S, Markman M, et al.: Long-term survival of advanced refractory ovarian carcinoma patients with small-volume disease treated with intraperitoneal chemotherapy. J Clin Oncol 5 (10): 1607-12, 1987.
- Alberts DS, Liu PY, Hannigan EV, et al.: Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335 (26): 1950-5, 1996.
- Markman M, Bundy BN, Alberts DS, et al.: Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 19 (4): 1001-7, 2001.
- Tewari D, Java JJ, Salani R, et al.: Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol 33 (13): 1460-6, 2015.
- Jaaback K, Johnson N: Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev (1): CD005340, 2006.
- Elit L, Oliver TK, Covens A, et al.: Intraperitoneal chemotherapy in the first-line treatment of women with stage III epithelial ovarian cancer: a systematic review with metaanalyses. Cancer 109 (4): 692-702, 2007.
- Walker JL, Brady MF, Wenzel L, et al.: Randomized Trial of Intravenous Versus Intraperitoneal Chemotherapy Plus Bevacizumab in Advanced Ovarian Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol 37 (16): 1380-1390, 2019.
- Vergote I, Tropé CG, Amant F, et al.: Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363 (10): 943-53, 2010.
- Kehoe S, Hook J, Nankivell M, et al.: Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 386 (9990): 249-57, 2015.
- Fagotti A, Ferrandina G, Vizzielli G, et al.: Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer 59: 22-33, 2016.
- Wright AA, Bohlke K, Armstrong DK, et al.: Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol Oncol 143 (1): 3-15, 2016.
- Sugarbaker PH: Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia 23 (5): 431-42, 2007.
- Koopman M, Antonini NF, Douma J, et al.: Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 370 (9582): 135-42, 2007.
- van Driel WJ, Koole SN, Sikorska K, et al.: Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med 378 (3): 230-240, 2018.
- Howell SB, Kirmani S, Lucas WE, et al.: A phase II trial of intraperitoneal cisplatin and etoposide for primary treatment of ovarian epithelial cancer. J Clin Oncol 8 (1): 137-45, 1990.
- Zivanovic O, Abramian A, Kullmann M, et al.: HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int J Cancer 136 (3): 699-708, 2015.
- Burger RA, Brady MF, Bookman MA, et al.: Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365 (26): 2473-83, 2011.
- Perren TJ, Swart AM, Pfisterer J, et al.: A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365 (26): 2484-96, 2011.
- Bryant HE, Schultz N, Thomas HD, et al.: Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434 (7035): 913-7, 2005.
- Farmer H, McCabe N, Lord CJ, et al.: Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434 (7035): 917-21, 2005.
- Moore K, Colombo N, Scambia G, et al.: Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 379 (26): 2495-2505, 2018.
- DiSilvestro P, Colombo N, Scambia G, et al.: Efficacy of Maintenance Olaparib for Patients With Newly Diagnosed Advanced Ovarian Cancer With a BRCA Mutation: Subgroup Analysis Findings From the SOLO1 Trial. J Clin Oncol 38 (30): 3528-3537, 2020.
- González-Martín A, Pothuri B, Vergote I, et al.: Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 381 (25): 2391-2402, 2019.
- Coleman RL, Fleming GF, Brady MF, et al.: Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med 381 (25): 2403-2415, 2019.
- Ray-Coquard I, Pautier P, Pignata S, et al.: Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med 381 (25): 2416-2428, 2019.
- Vergote IB, Jimeno A, Joly F, et al.: Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J Clin Oncol 32 (4): 320-6, 2014.
- Berek JS, Taylor PT, Gordon A, et al.: Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J Clin Oncol 22 (17): 3507-16, 2004.
- Verheijen RH, Massuger LF, Benigno BB, et al.: Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol 24 (4): 571-8, 2006.
- Markman M, Liu PY, Wilczynski S, et al.: Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol 21 (13): 2460-5, 2003.
- Pecorelli S, Favalli G, Gadducci A, et al.: Phase III trial of observation versus six courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after six courses of paclitaxel/platinum-based chemotherapy: final results of the After-6 protocol 1. J Clin Oncol 27 (28): 4642-8, 2009.
- Monk BJ, Colombo N, Oza AM, et al.: Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol 22 (9): 1275-1289, 2021.
- Moore KN, Bookman M, Sehouli J, et al.: Atezolizumab, Bevacizumab, and Chemotherapy for Newly Diagnosed Stage III or IV Ovarian Cancer: Placebo-Controlled Randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol 39 (17): 1842-1855, 2021.
Tratamiento del cáncer epitelial de ovario, cáncer de trompas de Falopio y cáncer primario de peritoneo recidivantes o crónicos
Alrededor del 80 % de las pacientes con diagnóstico de cáncer epitelial de ovario, cáncer de trompas de Falopio (CTF) y cáncer primario de peritoneo (CPP) sufrirán una recaída después de recibir quimioterapia de primera línea con derivados del platino y taxano, y se pueden beneficiar de tratamientos posteriores. Ya no se lleva a cabo la detección temprana de enfermedad crónica con laparotomías de segunda exploración después de completar el tratamiento de primera línea. Cuando se compararon de manera informal los desenlaces en (50 %) de las instituciones que practican dichos procedimientos con los de instituciones que no los utilizan, aumentó la falta de apoyo para la realización de laparotomías de segunda exploración. Esto se confirmó en el ensayo del Gynecologic Oncology Group (GOG) GOG-0158.
A pesar de esto, se adoptó casi universalmente la práctica de un seguimiento minucioso mediante la medición de la concentración del antígeno del cáncer 125 (CA-125) en intervalos de 1 a 3 meses de las pacientes que finalizan el tratamiento. En las pacientes con remisión clínica completa, el método más común para detectar la enfermedad que con el tiempo presentará una recaída clínica es el seguimiento de los aumentos de CA-125 a partir del tratamiento inicial.
En un ensayo clínico, se abordó el tratamiento basado en aumentos anormales en el CA-125, en ausencia de síntomas o pruebas de imágenes de la enfermedad.
Evidencia (iniciación temprana vs. iniciación diferida del tratamiento):
- En un ensayo del Medical Research Council (MRC) y la European Organisation for Research and Treatment of Cancer (MRC-OV05), se examinaron las consecuencias de instituir un tratamiento temprano ante una concentración elevada de CA-125 versus un tratamiento diferido hasta que aparecieran síntomas clínicos. Se inscribieron pacientes en remisión clínica completa luego de recibir quimioterapia con derivados del platino y se les hizo un seguimiento solo con control de las concentraciones de CA-125 y visitas clínicas. Cuando se detectó una elevación doble sobre el rango normal, las pacientes se asignaron al azar a recibir el resultado y tratamiento temprano de la recidiva versus continuar con el enmascaramiento y el tratamiento una vez que se presentaran signos y síntomas indicativos de recaída clínica. El número de pacientes asignadas al azar debía exceder a 500 para rendir un desenlace superior de supervivencia a los 2 años con administración temprana del tratamiento; esto exigió 1400 inscripciones entre mayo de 1996 y agosto de 2005.
- Entre las 1442 pacientes inscritas, el 29 % continuó sin ninguna prueba de recaída; sin embargo, en el 19 % de las pacientes, la concentración de CA-125 no informó sobre una recaída clínica, o sobre un duplicado simultáneo con la recaída clínica.
- Las pacientes tenían enfermedad en estadio III y estadio IV en un 67 % de los casos; no obstante, estos estadios representaron el 80 % de las pacientes que, con un aumento de dos veces o más de concentración de CA-125, posteriormente se asignaron al azar.
- La mediana de supervivencia de todas las pacientes inscritas fue de 70,8 meses.
- La mediana de supervivencia de las pacientes asignadas al azar a un tratamiento temprano (n = 265) fue 25,7 meses comparada con 27,1 meses para aquellas pacientes en el grupo de tratamiento diferido (n = 264) (coeficiente de riesgos instantáneos [CRI], 0,98; intervalo de confianza [IC] 95 %, 0,8–1,2).
- La mediana de demora para administrar quimioterapia de segunda línea fue de 4,8 meses y la mediana de demora en administrar quimioterapia de tercera línea fue de 4,6 meses. Los tratamientos con quimioterapia de segunda línea fueron comparables entre los dos grupos (en su mayoría a base de derivados del platino o taxanos), mientras que los tratamientos de tercera línea se administraron con menos frecuencia al grupo de tratamiento diferido.
- La conclusión del estudio fue que no hubo beneficio de la detección temprana de la enfermedad mediante la medición del CA-125. Este hallazgo concuerda con el fracaso de las cirugías de segunda exploración para proporcionar mejores desenlaces después de la detección temprana de una enfermedad crónica.
En una evaluación de la calidad de vida que acompaña este estudio, se encontró un efecto perjudicial del tratamiento temprano cuando se comparó con la espera de la presentación de signos y síntomas.
El efecto muy bajo de estos hallazgos en los patrones de vigilancia de CA-125 durante más de una década en cinco U.S. Cancer Centers fue decepcionante. La vigilancia de las concentraciones de CA-125 durante el seguimiento se usó para distinguir a pacientes con recidivas sensibles a los derivados del platino de las pacientes con recidivas resistentes a los derivados del platino, y desempeña una función en la identificación de pacientes aptas para una citorreducción secundaria, aunque esta estrategia aún se debe confirmar mediante un ensayo aleatorizado.
Opciones de tratamiento del cáncer epitelial de ovario, cáncer de trompas de Falopio y cáncer primario de peritoneo recidivantes o crónicos
Las opciones de tratamiento con fármacos para las pacientes con enfermedad recidivante se subdividen de la siguiente forma:
- Recidiva sensible a los derivados del platino: para pacientes cuya enfermedad recidiva más de 6 meses después de terminar la inducción, se debe considerar la repetición del tratamiento con un derivado del platino o una combinación de estos fármacos como el carboplatino (consultar el Cuadro 8).
- Recidiva con resistencia primaria o secundaria a los derivados del platino: para aquellas pacientes cuya enfermedad avanza antes de terminar la terapia de inducción (resistencia primaria a los derivados del platino) o dentro de los 6 meses de terminar la misma (resistencia secundaria a los derivados del platino), la terapia con derivados del platino por lo general no es útil como parte del plan de tratamiento. Se debe considerar la participación en ensayos clínicos.
Otros fármacos que exhibieron actividad en ensayos de fase II se enumeran en el Cuadro 10 y es posible usarlos solos o en combinación con otros fármacos; sin embargo, tales tratamientos se administran mejor en el entorno de ensayos prospectivos.
A veces se utiliza citorreducción; esta intervención está en estudio en el entorno de ensayos clínicos aleatorizados (por ejemplo, GOG-0213 [NCT00565851], DESKTOP III [NCT01166737], y SOC 1 [NCT01611766]). El ensayo SOC 1 se publicó con datos preliminares de supervivencia. Los criterios de idoneidad fueron diferentes en los ensayos. Solo el 67 % de las pacientes se sometieron a resecciones macroscópicas completas en el ensayo GOG-0213, en comparación con el 77 % de las pacientes en el ensayo SOC 1, y el 75 % de las pacientes en el ensayo DESKTOP III. El ensayo Dutch SOCcer terminó de manera prematura en 2015 porque solo se inscribieron 27 de las 230 pacientes previstas (12 %) durante 5 años. En el ensayo del GOG la meta de inscripción fue de 485 pacientes con el fin de estudiar la función de la cirugía, esto demoró casi 10 años.
No se ha definido la función de la radioterapia para pacientes con cáncer de ovario recidivante.
Recidiva sensible al platino
Regímenes de quimioterapia que contienen platino
En el Cuadro 8 se presentan los regímenes de quimioterapia usados en la primera recaída para tratar la recidiva del cáncer de ovario sensible a los derivados del platino.
| Elegibilidad (meses) desde el final del tratamiento inicial | Régimen | Número de pacientes | Régimen comparativo | Comentarios sobre los resultados (meses) |
|---|---|---|---|---|
| CRI = cociente de riesgos instantáneos; SG = supervivencia general; SSP = supervivencia sin progresión; DLP = doxorrubicina liposomal pegilada. | ||||
| aLa trabectedina se aprobó para usar en el tratamiento de cáncer de ovario recidivante en Europa y Canadá. | ||||
| bLos datos sobre la SG no eran definitivos en el momento de la publicación. | ||||
| cP< 0,0001. | ||||
| dP = 0,012. | ||||
| eCRI, 0,51; P = 0,0001. | ||||
| Utilizados con más frecuencia | ||||
| Sensible a los derivados del platino (>6) | Cisplatino o carboplatino y paclitaxel | 802 | Otra monoterapia sin taxanos y derivados del platino | SSP 11 vs. 9; SG 24 vs. 19 |
| Sensible a los derivados del platino (>6) | Carboplatino y gemcitabina | 356 | Carboplatino | SSP 8,6 vs. 5,8; SG 18 vs. 17 |
| Sensible a los derivados del platino (>6) | Carboplatino y DLP | 976 | Paclitaxel y carboplatino | SSP 11,3 vs. 9,4; SG 30,7 vs. 33,0 |
| Otros regímenes | ||||
| Sensible a los derivados del platino (>6) | Carboplatino y epirrubicina | 190 | Carboplatino | Potenciado para diferencias en respuestas; SG 17 vs. 15 |
| Sensible a los derivados del platino (≥12) | Cisplatino, doxorrubicina y ciclofosfamida | 97 | Paclitaxel | SSP 15,7 vs. 9; SG 34,7 vs. 25,8 |
| Sensible + resistente a los derivados del platino | DLP y trabectedinaa | 672 | DLP | SSP 7,3 vs. 5,8; SG 20,5 vs. 19,4 b |
| Sensible a los derivados del platino | Paclitaxel y carboplatino | 674 | Paclitaxel, carboplatino y bevacizumab | SSP 10,4 vs. 13,8c; SG 37,4 vs. 42,2 |
| Sensible a los derivados del platino | Carboplatino, DLP y bevacizumab | 682 | Carboplatino, gemcitabina y bevacizumab | SSP 12,4 vs. 11,3d, SG 31,9 vs. 27,8 |
| Sensible a los derivados del platino | Carboplatino y paclitaxel, o gemcitabina, o DLP | 406 | Díadas iguales y bevacizumab | SSP 8,8 vs. 11,8e, muertes 68 vs. 79, SG 27,1 vs. 26,7 |
A partir de una mejora de la supervivencia con etopósido o fluorouracilo, en 1987 se aprobó el carboplatino para el tratamiento de pacientes con cáncer de ovario cuya enfermedad recidivó después del tratamiento con cisplatino. En un ensayo aleatorizado de fase ll con paclitaxel, un medicamento de segunda línea de uso actual, la combinación que contiene cisplatino y doxorrubicina además de ciclofosfamida, rindió un resultado superior para la supervivencia. En este y otros ensayos posteriores (consultar el Cuadro 8), se ha consolidado el uso del carboplatino como tratamiento principal de pacientes con recidivas sensibles a los derivados del platino. En ocasiones, se usa cisplatino, en especial en combinación con otros fármacos, porque produce menor mielodepresión; sin embargo, esta ventaja sobre el carboplatino se contrarresta por la mayor intolerancia en la paciente.
El oxaliplatino, que si bien se introdujo al comienzo con la esperanza de vencer la resistencia a los derivados del platino, exhibe mayor actividad en pacientes sensibles al platino; sin embargo, no se comparó con el carboplatino solo o en combinaciones.
Con todos los derivados del platino, el resultado suele ser mejor mientras mayor haya sido el intervalo inicial sin recidiva desde los regímenes iniciales que contienen platino. En consecuencia, las pacientes con recidivas sensibles a los derivados del platino que recayeron en un plazo de 1 año se incluyeron en ensayos con fármacos sin platino. En uno de tales ensayos, en el que se comparó la doxorrubicina liposomal pegilada con el topotecán, el subconjunto de pacientes sensibles a los derivados del platino tuvo mejores desenlaces con cualquiera de los fármacos (en particular con la doxorrubicina liposomal pegilada) que la cohorte resistente al platino.
En varios ensayos aleatorizados se consideró si el uso de un derivado del platino combinado con otros fármacos quimioterapéuticos es superior a los fármacos solos (consultar el Cuadro 8).
Evidencia (derivado del platino combinado con otros fármacos quimioterapéuticos):
- En un análisis de datos en el que se examinaron en forma conjunta los resultados de ensayos llevados a cabo por investigadores del MRC/Arbeitsgemeinschaft Gynaekologische Onkologie (MRC/AGO) y el International Collaborative Ovarian Neoplasm (ICON) (ICON4), se observaron los siguientes resultados:[Nivel de evidencia A1]
- Una combinación de un derivado del platino con paclitaxel rindió tasas de respuesta superiores de supervivencia sin progresión (SSP) y supervivencia general (SG), en comparación con el carboplatino como fármaco único u otras combinaciones con derivados del platino como controles.
- Se comparó un derivado del platino con el paclitaxel con varios regímenes de control, aunque el 71 % contenían carboplatino como fármaco único y el 80 % contenían carboplatino paclitaxel. La SSP prolongada (CRI, 0,76; IC 95 %, 0,66–0,89; P = 0,004) y la SG (CRI, 0,82; IC 95 %, 0,69–0,97; P = 0,023) mejoraron en el grupo de derivado del platino y paclitaxel.; [Nivel de evidencia A1]
- En el AGO, se había estudiado antes la combinación de epirrubicina y carboplatino en comparación con el carboplatino solo y no se encontraron diferencias significativas en los desenlaces.
- En un metanálisis de cinco ensayos (tres de los cuales se mencionan en el Cuadro 8), cuatro con revisión independiente de datos de pacientes, se apoya el uso de fármacos derivados del platino combinados con otros fármacos activos en lugar del carboplatino solo para pacientes de cáncer de ovario recidivante sensible al platino.
- En otro ensayo realizado por grupos europeos y canadienses, se estudió la gemcitabina y el carboplatino en comparación con el carboplatino.
- La SSP a los 8,6 meses con la combinación fue significativamente superior a la del carboplatino solo (CRI, 0,72; IC 95 %, 0,58–0,90; P = 0,003).[Nivel de evidencia B1]
- El estudio no tuvo potencia estadística suficiente para detectar diferencias significativas en la SG; la mediana de supervivencia en los dos grupos fue de 18 meses (CRI, 0,96; IC, 0,75–1,23; P = 0,73).
- En un ensayo en fase lll, carboplatino más doxorrubicina liposomal pegilada se comparó con el carboplatino más paclitaxel en pacientes con recidivas sensible a los derivados del platino (>6 meses). El criterio principal de valoración fue la SSP.
- La mediana de SSP para el grupo de carboplatino más doxorrubicina liposomal pegilada fue de 11,3 versus 9,4 meses para el grupo de carboplatino más paclitaxel (CRI, 0,823; IC 95 %, 0,72–0,94; P = 0,005).[Nivel de evidencia B1]
- El seguimiento a largo plazo no reveló diferencias en las tasas de SG entre los dos grupos (30,7 meses para carboplatino y doxorrubicina liposomal pegilada vs. 33,0 meses para carboplatino y paclitaxel).
- El grupo de carboplatino más paclitaxel se relacionó con un aumento grave de neutropenia, alopecia, neuropatía y reacción alérgica. El grupo de carboplatino más doxorrubicina liposomal pegilada se relacionó con un aumento de trombocitopenia grave, náuseas y eritrodisestesia palmoplantar.
Dado su perfil tóxico y ausencia de inferioridad ante el régimen estándar, el carboplatino más doxorrubicina liposomal pegilada es una opción importante para las pacientes con recidiva sensible a los derivados del platino.
El carboplatino más paclitaxel se ha considerado el régimen estándar para las recidivas sensibles a los derivados del platino en ausencia de efectos neurológicos residuales. En el ensayo GOG-0213, se está comparando este régimen con el grupo experimental al que se le añade bevacizumab al carboplatino más paclitaxel.
Bevacizumab, otros fármacos dirigidos e inhibidores de la poli (ADP-ribosa)–polimerasa (PARP), con quimioterapia o sin esta
Evidencia (bevacizumab con gemcitabina/carboplatino):
- En el Ovarian Cancer Study Comparing Efficacy and Safety of Chemotherapy and Anti-Angiogenic Therapy in Platinum-Sensitive Recurrent Diseases (OCEANS [NCT00434642]), se evaluó el papel de bevacizumab en el tratamiento de la recidiva sensible a los derivados del platino (para obtener información de otros ensayos en este entorno, consultar el Cuadro 8). En este ensayo controlado con placebo, con enmascaramiento doble de fase III sobre quimioterapia (gemcitabina y carboplatino) con bevacizumab o sin este para el cáncer epitelial de ovario recidivante, el CTF o el CPP, se asignó al azar a 242 pacientes a cada grupo. En contraste con los estudios de primera línea, se permitió que el tratamiento continuara más allá de 6 ciclos hasta 10 ciclos en las pacientes que respondieron, pero no se administró terapia de mantenimiento.
- Se dará a conocer un análisis posterior cuando se completen los datos de supervivencia; sin embargo, en el momento de la publicación, las diferencias en la mediana de SG no eran evidentes, y el 31 % de las pacientes habían pasado del grupo de placebo al de bevacizumab.
- La mediana de SSP de las pacientes que recibieron bevacizumab fue de 12,4 versus 8,4 meses de las pacientes que recibieron placebo.
- El CRI para el efecto de bevacizumab en la progresión de la enfermedad en las pacientes asignadas al grupo de bevacizumab en comparación con el placebo fue de 0,484 (IC 95 %, 0,388–0,605; P< 0,0001).
- Las respuestas objetivas a la quimioterapia aumentaron cuando se combinaron con bevacizumab (78,5 % vs. 57,4 %; P< 0,0001).
- Los efectos tóxicos relacionados con el bevacizumab, como hipertensión y proteinuria fueron más evidentes que en los ensayos de primera línea, pero los problemas de inocuidad que se temía, como perforaciones gastrointestinales, no se presentaron durante el estudio.
- La interrupción del tratamiento por efectos adversos fue más frecuente con bevacizumab (n = 55 vs. n = 12 para el placebo), pero menos pacientes abandonaron el tratamiento debido a la progresión de la enfermedad (n = 104 para bevacizumab vs. n = 160 para el placebo).
Evidencia (bevacizumab añadido al carboplatino o a las díadas de carboplatino):
- El NRG Oncology Group o el grupo National Clinical Trials Network, un trabajo de investigación combinado del National Surgical Adjuvant Breast and Bowel Project, el Radiation Therapy Oncology Group y el GOG (GOG-0213 [NCT00565851]), evaluaron tanto la función de la citorreducción quirúrgica como la adición de bevacizumab para la inducción y el mantenimiento en mujeres con recidivas de cáncer de ovario sensibles al platino.[Nivel de evidencia A1] La parte no quirúrgica de GOG-0213 tuvo una potencia del 81 % para un verdadero CRI de 0,75; en esta parte del ensayo se inscribieron 674 mujeres entre diciembre de 2007 y agosto de 2011. El análisis publicado tuvo lugar después de una mediana de seguimiento de más de 4 años.
- La SG no fue significativamente diferente: 37,3 meses (IC 95 %, 32,6–39,7) versus 42,2 meses (IC 95 %, 37,7–46,2).
- El criterio secundario de valoración de la mediana de SSP favoreció significativamente la adición de bevacizumab: 10,4 meses (IC 95 %, 9,7–11) para la quimioterapia sola versus 13,8 meses (IC 95 %, 13,0–14,7).
- Bevacizumab (15 mg/kg cada 3 semanas) con quimioterapia y su uso durante el mantenimiento condujo a un exceso de efectos adversos de grados 3 and 4 (8 % para la quimioterapia sola vs. 30 %), cualquier sangrado (12 % vs. 42 %) e hipertensión (3 % vs. 41 %).
- En un ensayo aleatorizado sin enmascaramiento de fase III (MITO16b/MANGO-OV2/ENGOT-ov17 [NCT01802749]), los investigadores inscribieron a 406 pacientes en el momento de la primera recidiva o progresión, por lo menos 6 meses después de recibir un tratamiento de primera línea a base de derivados del platino, que incluyó bevacizumab durante la inducción o el mantenimiento. En el ensayo se comparó el uso de s de carboplatino con bevacizumab o sin este como tratamiento estándar en pacientes con recidiva sensible a los derivados del platino. Los programas de dosificación variaron según la díada, pero en todos los casos el bevacizumab se administró en dosis de 5 mg/kg/semana. En el estudio, 21 pacientes recibieron carboplatino y paclitaxel; 22 pacientes recibieron carboplatino, paclitaxel y bevacizumab; 99 pacientes recibieron cisplatino y gemcitabina; 98 pacientes recibieron cisplatino, gemcitabina y bevacizumab; 83 pacientes recibieron doxorrubicina pegilada liposomal; y 83 pacientes recibieron doxorrubicina pegilada liposomal con bevacizumab. Las pacientes se inscribieron en el ensayo entre diciembre de 2013 y noviembre de 2016. El criterio principal de valoración fue la SSP evaluada por el investigador, se intentó reducir el CRI de recidiva a 0,67 (potencia de 90 % y prueba bilateral α = 0,05). Los criterios secundarios de valoración fueron la tasa de SG (desde el momento de la aleatorización hasta la muerte por cualquier causa) y las tasas de respuesta objetiva según los Response Evaluation Criteria in Solid Tumors (RECIST).
- La mediana de SSP fue de 8,8 meses (IC 95 %, 8,4–9,3) en las pacientes del grupo de quimioterapia estándar versus 11,8 meses (CRI, 0,51; IC 95 %, 10,8–12,9) en las pacientes del grupo de quimioterapia y bevacizumab.[Nivel de evidencia B1]
- La SG no fue diferente entre los dos grupos: la mediana de SG fue de 27,1 meses en las pacientes del grupo de tratamiento estándar versus 26,7 meses en las pacientes del grupo de quimioterapia y bevacizumab.
- En el análisis de la tasa de respuesta objetiva se determinó que 71 de 143 pacientes en el grupo estándar y 90 de 130 pacientes en el grupo de quimioterapia y bevacizumab presentaron respuestas completas o parciales.
- La población de inocuidad abarcó a 200 pacientes del grupo estándar y 201 pacientes del grupo de quimioterapia y bevacizumab. Se registraron múltiples efectos adversos graves en ambos grupos: 61 efectos en 41 pacientes del grupo estándar y 76 efectos en 52 pacientes del grupo de quimioterapia y bevacizumab.
- La hipertensión fue un efecto adverso grave. En el grupo estándar, el 93 % de las pacientes presentaron hipertensión: en 166 pacientes fue de grado 1 o 2, y en 20 pacientes fue de grado 3. En el grupo de quimioterapia y bevacizumab, el 98 % de las pacientes presentaron hipertensión: en 139 pacientes fue de grado 1 o 2, y en 58 pacientes fue de grado 3.
- La proteinuria y la epistaxis también fueron efectos adversos de grado 1 o 2 en el grupo de pacientes de quimioterapia y bevacizumab.
En el estudio se demostró que la adición de bevacizumab mejoró la SSP pero no conllevó beneficio de la SG, y aumentó la toxicidad. Este estudio precede la incorporación de los inhibidores de PARP en los ensayos de fase III en pacientes con recidiva sensible a los derivados del platino. En un análisis de un subgrupo de este estudio de pacientes con mutaciones en BRCA, se documentaron mutaciones deletéreas solo en 23 pacientes del grupo estándar y en 30 pacientes del grupo de quimioterapia y bevacizumab. Estos datos destacan la importancia de la evolución del tratamiento dirigido que ocurrió durante el estudio y después.
Evidencia (bevacizumab y doxorrubicina liposomal pegilada/carboplatino vs. bevacizumab y gemcitabina/carboplatino):
Para obtener información sobre los resultados de ensayos clínicos en los que se usaron gemcitabina/carboplatino, consultar el Cuadro 8. Los efectos adversos difirieron entre los dos comparadores, pero los efectos adversos graves fueron del 10 % con doxorrubicina/carboplatino liposomal pegilado y del 9 % con gemcitabina/carboplatino. En particular, una crisis hipertensiva, presuntamente relacionada con el bevacizumab, se presentó en cinco pacientes después de recibir doxorrubicina liposomal pegilada/carboplatino y en tres pacientes después de recibir gemcitabina/carboplatino.
Evidencia (inhibidores de PARP, con antiangiogénicos o sin estos):
PARP es una familia de enzimas que participan en la reparación por escisión de bases de las roturas monocatenarias del ADN. En pacientes con deficiencia en la recombinación homóloga, incluso pacientes con mutaciones en la línea germinal de BRCA1 o BRCA2 (gBRCA) o con tumores que exhiben deficiencia en la recombinación homóloga fuera de la línea germinal, la inhibición de PARP produce roturas bicatenarias del ADN. Los mecanismos humanos de reparación del ADN descansan en su mayoría en una copia intacta del gen; las células con roturas bicatenarias suelen ser el objetivo de la muerte celular. Esta susceptibilidad a la inhibición de PARP de las células con deficiencia del BRCA o una mutación en el BRCA ha estimulado el desarrollo clínico de esta clase de sustancias. Debido a que una característica de la deficiencia en la recombinación homóloga es la sensibilidad a los compuestos de platino, se espera que una población de pacientes sensibles a los derivados del platino exhiba mayor deficiencia en la recombinación homóloga, y es muy probable que se beneficie de la inhibición de PARP. Se ha estudiado desde 2005 el uso de olaparib, cuando en un estudio de fase I participaron mujeres con cáncer de ovario de las que se conocía el estado de ser portadoras de una mutación en BRCA. Debido a las respuestas objetivas en este ensayo inicial, se ha estudiado el olaparib, y posteriormente el rucaparib y el niraparib, después de varias líneas de tratamiento para la recidiva; estos estudios condujeron a una aprobación inicial del olaparib y, luego, del rucaparib y el niraparib, como se presentan en el Cuadro 9.
| Fármaco | Potencia de captura de PARP | Indicaciones aprobadas por la FDA | Dosis | Ensayos clave | Efectos tóxicos | Otras características |
|---|---|---|---|---|---|---|
| CPP = cáncer primario de peritoneo; CTF = cáncer de trompas de Falopio; DRH = deficiencia de la recombinación homóloga; FDA = Administración de Alimentos y Medicamentos de los Estados Unidos; gBRCA = línea germinal de BRCA; LMA = leucemia mieloide aguda; PARP = poli(ADP-ribosa)–polimerasa; PB = por boca; RC = respuesta completa; RP = respuesta parcial; SMD = síndrome mielodisplásico; tBRCA = tumor BRCA. | ||||||
| Olaparib | Intermedia | Mantenimiento del cáncer epitelial de ovario recidivante, CTF o CPP en pacientes con RC o RP a la quimioterapia con derivados del platino, independientemente del estado del BRCA | Dos dosis diarias de comprimidos de 300 mg (remplazando las cápsulas de 400 mg) | Estudio 19, Estudio 42, SOLO2 (NCT01874353), SOLO1 (NCT01844986) (actualizado en octubre de 2018), otros en curso | Náuseas, fatiga, mielodepresión (en especial, anemia), dolor abdominal; casos raros de SMD/LMA | Prueba diagnóstica acompañante para evaluar los efectos nocivos de las mutaciones en gBRCA cuando se usa olaparib para el tratamiento (no para el mantenimiento) |
| Rucaparib | Baja-intermedia | Mantenimiento del cancer epitelial de ovario, CTF o CPP recidivantes para pacientes con RC o RP a la quimioterapia con derivados del platino, independientemente del estado del BRCA | Dos dosis diarias de 600 mg PB | Estudio 10, ARIEL2 (NCT01891344), ARIEL3 (NCT01968213), ARIEL4 (NCT02855944) (en curso), muchos otros en curso | Náuseas, fatiga, aumento de enzimas hepáticas, mielodepresión (en especial, anemia), dolor abdominal; casos raros de SMD/LMA | Prueba diagnóstica acompañante para evaluar los efectos nocivos de las mutaciones en gBRCA cuando se usa rucaparib para el tratamiento (no para el mantenimiento); hay ensayos de DRH que lo usan , pero todavía no está aprobado para ese uso |
| Niraparib | Intermedia-alta | Mantenimiento para pacientes de cáncer epitelial de ovario, CTF o CPP recidivantes para pacientes con RC o RP a la quimioterapia con derivados del platino, independientemente del estado del BRCA | 1 dosis diaria de 300 mg PB | NOVA (NCT01847274), QUADRA (NCT02354586), TOPACIO (NCT02657889) (en curso), PRIMA (NCT02655016) (en curso), otros en curso | Náuseas, fatiga, estreñimiento, hipertensión, mielodepresión (en especial ↓ de plaquetas); casos raros de SMD/LMA | En ensayos clínicos clave se usaron pruebas de DRH, pero todavía no se aprobó para este uso |
| Veliparib | Baja | Ninguna todavía | Ensayos en curso combinando veliparib con quimioterapia | |||
| Talazoparib | Alta | Ninguna todavía | Ensayos en curso, algunos con inmunoterapia | |||
- En un ensayo aleatorizado de fase ll, con enmascaramiento doble y controlado con placebo de la terapia de mantenimiento con olaparib, se consideró aptas a las pacientes de cáncer de ovario seroso de grado alto sensible a los derivados del platino. Las pacientes se asignaron al azar para recibir olaparib (400 mg dos veces por día) o placebo. La mutación tipo gBRCA1 o gBRCA2 no fue un requisito para la participación; sin embargo, se sabía que el 23 % de las pacientes del grupo experimental y el 22 % de las pacientes del grupo de placebo tenían una mutación en BRCA1 o BRCA2. El criterio principal de valoración fue la SSP.[Nivel de evidencia B1]
- La mediana de SSP fue de 8,4 meses en el grupo de olaparib versus 4,8 meses en las pacientes del grupo de placebo (CRI, 0,35; IC 95 %, 0,25–0,49; P< 0,001).
- Como se hizo notar en un informe actualizado, no hubo diferencia en la SG entre los dos grupos.
- Los efectos adversos más frecuentes en el grupo de olaparib fueron náusea, fatiga, vómitos y anemia.
- En el ensayo de fase III SOLO2 (NCT01874353) aleatorizado, con enmascaramiento doble y controlado con placebo, se evaluaron los comprimidos de olaparib (a diferencia de la formulación anterior en cápsula) en pacientes de cáncer seroso de grado alto o endometrioide, CPP o CTF. Las pacientes tuvieron recaídas sensibles a los derivados del platino y se preseleccionaron de acuerdo con las mutaciones en BRCA1/BRCA2.[Nivel de evidencia B1] Se realizó la estratificación de acuerdo con la respuesta (completa vs. parcial) a intervalos previos con derivados del platino y a intervalos sin este (>6–12 vs. >12 meses), y una distribución aleatoria en proporción 2:1 a olaparib en dosis de150-mg dos veces por día o un placebo idéntico. De las 295 pacientes aptas inscritas, se asignó a 196 a olaparib y a 99 al placebo.
- El criterio principal de valoración fue la SSP y esta favoreció significativamente al olaparib (19,1 meses [IC 95 %, 16,3–25,7]) en comparación con el placebo (5,5 meses [intervalo, 5,2–5,8]; CRI, 0,30 [IC 95 %, 0,22–0,41]; P< 0,0001).
- De las pacientes que recibieron olaparib, el 18 % experimentaron efectos adversos graves y, en el grupo que recibió placebo, el 8 % los experimentaron. Los efectos adversos fueron, en su mayoría, anemia, dolor abdominal y obstrucción intestinal.
- En este ensayo, se realizó una evaluación integral de las mediciones de la calidad de vida relacionada con la salud en pacientes que recibieron olaparib comparados con las pacientes que recibieron un placebo. Con el uso del puntaje del Trial Outcome Index en un análisis de cambios preespecificados, se observó que se cumplieron varias mediciones. Además, el tiempo sin síntomas importantes de toxicidad y la SSP ajustada por la calidad fueron más prolongados en las pacientes tratadas con olaparib. Estas evaluaciones complementan otras mediciones, como el tiempo hasta el primer tratamiento y el tiempo hasta el tratamiento subsiguiente o la muerte que se han buscado para complementar la SSP como criterio principal de valoración para la aprobación del medicamento.
- En el ensayo SOLO3 [NCT00628251], un estudio de fase III en el que se usó una aleatorización 2:1, se compararon los comprimidos de olaparib con la quimioterapia sin derivados del platino elegida por el médico (doxorrubicina liposomal pegilada, paclitaxel semanal, gemcitabina o topotecán) para el tratamiento del cáncer de ovario recidivante sensible a derivados del platino y mutaciones en BRCA1/BRCA2. En un estudio previo aleatorizado de fase II de concentraciones de dosis de comprimidos de olaparib de 200 o 400 mg dos veces por día versus doxorrubicina liposomal pegilada, no se pudo encontrar una ventaja sobre la quimioterapia. En el ensayo SOLO3, se asignó a 178 pacientes para recibir olaparib y a 88 pacientes para recibir la quimioterapia elegida por el médico. El criterio principal de valoración fue la tasa de respuesta general.
- De las 151 pacientes evaluadas, 109 exhibieron respuestas objetivas al olaparib (14 respuestas completas), mientras que de 72 pacientes evaluadas, 37 exhibieron respuestas objetivas a la quimioterapia (2 respuestas completas).
- En el análisis final, no hubo diferencia significativa en la SG. La mediana de SG fue de 34,9 meses en las pacientes que recibieron olaparib y de 32,9 meses en las pacientes que recibieron quimioterapia (CRI, 1,07; P = 0,71).
- En un análisis de subgrupos de pacientes tratadas con al menos 3 líneas de quimioterapia, se observó un perjuicio en la sobrevivencia. Entre estas pacientes, la mediana de la SG fue de 29,9 meses en el grupo de olaparib y de 39,4 meses en el grupo de quimioterapia (CRI, 1,33; IC 95 %, 0,84–2,18).
- Los efectos adversos concordaron con los perfiles de toxicidad del olaparib y los comparadores de quimioterapia; sin embargo, en la población pretratada, 4 pacientes asignadas a recibir olaparib y 3 pacientes asignadas a recibir quimioterapia presentaron leucemia mieloide aguda (AML)/mielodisplasia; también se presentaron neoplasias malignas primarias en 3 pacientes asignadas a recibir olaparib.[Nivel de evidencia B1]
- En agosto 26 de 2022, AstraZeneca retiró la indicación de olaparib en monoterapia para el tratamiento de pacientes con cáncer de ovario con mutaciones germinales deletéreas en BRCA que recibieron al menos tres líneas previas de quimioterapia.
- El rucaparib se sometió a una evaluación de fase II en el estudio ARIEL2 (NCT01891344) sin enmascaramiento. Se inscribieron 206 pacientes de las cuales 204 ya estaban recibiendo el fármaco (192 ya estaban en subgrupos clasificables) y ellas experimentaron recidivas sensibles a los derivados del platino de grado alto entre octubre de 2013 y noviembre de 2014.[Nivel de evidencia C2] Se estudiaron los tres subgrupos siguientes de deficiencia de recombinación homóloga predefinidos de acuerdo con el análisis mutacional del tumor:
- BRCA mutado (efectos nocivos genéticos o somáticos) (n = 40).
- BRCA tipo natural y pérdida de heterocigosis (PDH) alta cuantificada mediante un análisis de secuenciación de última generación (LOH alta) (n = 82).
- BRCA tipo natural y (PDH baja) (n = 70).
- La mediana de SSP después de comenzar el tratamiento con rucaparib de las pacientes con mutaciones perniciosas en BRCA fue de 12,8 meses (IC 95 %, 9,0–14,7); para aquellas con PDH alto fue de 5,7 meses (intervalo, 5,3–7,6 meses) y para aquellas con PDH bajo fue de 5,2 meses (intervalo, 3,6–5,5 meses).
- En el estudio también se observó que la mutación en BRCA y su estado de metilación así como la recombinación de otros genes homólogos, como RAD51C, se puede relacionar con una PDH genómica alta en los tumores con BRCA de tipo natural que confiere tasas más altas de respuesta al rucaparib que las observadas en pacientes con PDH genómica baja.
- El rucaparib se evaluó más tarde para la terapia de mantenimiento después de la respuesta a la terapia con derivados del platino en un ensayo de fase III aleatorizado, con enmascaramiento doble y controlado con placebo (ARIEL3 [NCT01968213]). Para participar, las pacientes debían presentar carcinomas de grado alto tratados antes con por lo menos dos regímenes a base de derivados del platino y haber exhibido respuestas parciales o completas al último de estos regímenes. Entre abril de 2014 y julio de 2016 y en una proporción 2:1, se asignó a 375 pacientes a recibir rucaparib y a 189 pacientes a recibir un placebo.
- El criterio principal de valoración fue la SSP determinada por el investigador y se usó un procedimiento de reducción en las siguientes tres cohortes anidadas de tratamiento:
- Para las pacientes de las que se sabía que tenían mutaciones deletéreas en BRCA, somáticas o de la línea germinal , la SSP fue de 16,6 meses en el grupo de rucaparib (IC 95 %, 13,4–22,9) versus 5,4 meses en el grupo de placebo (IC 95 %, 3,4–6,7; CRI, 0,23; IC 95 %, 0,16–0,34; P< 0,0001).
- Para las pacientes con deficiencia en la recombinación homóloga, la SSP fue de 13,6 meses en el grupo de rucaparib (IC 95 %, 10,9–16,2) versus 5,4 meses en el grupo de placebo (IC 95 %, 5,1–5,6; CRI, 0,32; IC 95 %, 0,24–0,42; P< 0,00011).
- Para la población del grupo por intención de tratar, la SSP fue de 10,8 meses en el grupo de rucaparib (IC 95 %, 8,3–11,4) versus 5,4 meses en el grupo de placebo (IC 95 %, 5,3–5,5; CRI, 0,24–0,42; P < 0,0001.
- Los efectos adversos de grado 3 o más alto relacionados con el tratamiento en el grupo de rucaparib versus el grupo de placebo fueron principalmente anemia (19 % vs. 1 %) y aumento de la alanina–aminotransferasa o la aspartato–aminotransferasa (10 % vs. 0 %).
- En un análisis por intención de tratar actualizado de los resultados y la inocuidad para todas las cohortes, al cabo de una mediana de seguimiento de 28 meses, el rucaparib exhibió ventajas significativas y persistentes en términos de la SSP en comparación con un placebo (14,3 vs. 8,8 meses [CRI, 0,43; IC 95 %, 0,35−0,53]).[Nivel de evidencia B1]
- El tiempo transcurrido hasta el primer tratamiento siguiente, el tiempo hasta la progresión durante el tratamiento siguiente y el tiempo hasta comenzar un segundo tratamiento siguiente también mostraron valores significativos en favor del rucaparib.
- Se notificaron tres episodios de LMA o mielodisplasia relacionados con el tratamiento, y se registraron efectos adversos graves del tratamiento en el 22 % de las pacientes que recibieron el rucaparib versus el 11 % de las pacientes que recibieron el placebo.
- El efecto tóxico más común fue la anemia atribuida al rucaparib (22 % de las pacientes).
- En un análisis de este ensayo de la SSP ajustada por la calidad y el tiempo ajustado por la calidad sin síntomas ni toxicidad, se confirmó que rucaparib fue beneficioso en comparación con un placebo en todas las cohortes predefinidas.
- El criterio principal de valoración fue la SSP determinada por el investigador y se usó un procedimiento de reducción en las siguientes tres cohortes anidadas de tratamiento:
- En el ensayo ARIEL4 (NCT02855944), se evaluó el rucaparib versus quimioterapia en pacientes con cáncer epitelial de ovario de grado alto con mutación en BRCA tratadas con al menos dos líneas previas de quimioterapia.
- En la población por intención de tratar, la mediana de la SG fue de 19,4 meses en el grupo de rucaparib y de 25,4 meses en el grupo de quimioterapia (CRI, 1,31; IC 95 %, 1,00–1,73; P = 0,0507).
- En junio 10 de 2022, Clovis Oncology retiró la indicación del rucaparib en monoterapia para el tratamiento de pacientes de cáncer con mutación en BRCA tratadas con al menos dos líneas previas de quimioterapia.
- En el ensayo multicéntrico de fase II sin enmascaramiento con un solo grupo, QUADRA (NCT02354586), que se llevó a cabo desde 2015 hasta 2017, se estudió el niraparib como tratamiento de última línea para pacientes de cáncer de ovario. Se inscribió a 463 mujeres que se habían sometido a una mediana de 4 (intervalo, 3−5) regímenes previos (151 pacientes eran resistentes a los derivados del platino y 161 pacientes tenían resistencia primaria a los derivados del platino).
- En la población de eficacia primaria mensurable, 13 de 47 pacientes respondieron de acuerdo con los RECIST.[Nivel de evidencia C2]
- La deficiencia de recombinación homóloga fue un factor pronóstico de la respuesta.
- En septiembre 14 de 2022, GSK retiró la indicación de niraparib en monoterapia para la cuarta línea de tratamiento en pacientes con tumores epiteliales de ovario relacionados con deficiencia en la recombinación homóloga debido a que los fabricantes de olaparib y rucaparib encontraron una disminución de la supervivencia en pacientes que recibieron quimioterapia previa cuando se usó un inhibidor de PARP en monoterapia.
- El niraparib se evaluó más aún en un ensayo de fase III con enmascaramiento doble y controlado con placebo, en el que participaron 533 pacientes de cáncer de ovario seroso sensibles a los derivados del platino y predominantemente de grado alto se asignaron al azar en una proporción 2:1 a mantenimiento con niraparib oral o placebo y se les realizó seguimiento de la SSP, que era el criterio principal de valoración. Las pacientes se categorizaron de acuerdo a la presencia o ausencia de gBRCA o cáncer de ovario con deficiencia en la recombinación homóloga y sin BRCA o cáncer de ovario sin deficiencia en la recombinación homóloga y sin BRCA según pruebas de BRCAAnalysis (Myriad Genetics) de muestras del tumor y la sangre.
- Las pacientes que recibieron niraparib tuvieron una mediana de SSP significativamente más larga que aquellas que recibieron placebo.[Nivel de evidencia B1] Las comparaciones entre las categorías oscilaron entre un CRI de 0,27 para el cáncer con gBRCA (21,0 vs. 5,5 meses); CRI de 0,38 para el cáncer con deficiencia en la recombinación homóloga y sin BRCA (12,9 vs. 3,8 meses) y CRI de 0,45 para el cáncer sin deficiencia en la recombinación homóloga y sin BRCA (9,3 vs. 3,9 meses).
- Durante el estudio, se produjo la muerte del 16,1 % de las pacientes del grupo que recibió niraparib y del 19,3 % de las pacientes que recibieron placebo.
- Entre un tercio y la mitad de las pacientes habían recibido por lo menos tres líneas de tratamiento que incluyeron lo siguiente:
- Los efectos adversos de grados 3 o 4 que se trataron con modificaciones de las dosis mientras las pacientes recibían niraparib incluyeron trombocitopenia (33,8 % de las pacientes), anemia (25,3 %) y neutropenia (19,6 %).
- Otros excesos de efectos tóxicos graves en pacientes que recibieron niraparib se presentaron con dosis iniciales de 300 mg una vez por día e incluyeron fatiga (en 30 pacientes vs. 1 paciente en el grupo de placebo), hipertensión (en 30 pacientes vs. 4 pacientes en el grupo de placebo), náuseas (en 11 pacientes vs. 2 pacientes en el grupo de placebo) y vómitos (en 7 pacientes vs. 1 paciente en el grupo de placebo).
- En un análisis posterior del ensayo ENGOT-OV16/NOVA (NCT01847274) se actualizaron las SSP después del mantenimiento con niraparib o placebo de acuerdo con la mejor respuesta (respuesta parcial [RP] o respuesta completa [RC]) para la última quimioterapia con derivados del platino de pacientes con mutaciones en gBRCA y cohortes sin mutaciones en gBRCA.
- El CRI fue significativo para el niraparib versus placebo, y se observó en ambos grupos.
- No se observaron diferencias importantes en los desenlaces notificados por las pacientes.
- En un ensayo de fase III, aleatorizado con enmascaramiento doble y controlado con placebo, en el que se estudia el mantenimiento con niraparib para pacientes de cáncer de ovario en estadio avanzado con deficiencia en la recombinación homóloga luego de que logren una respuesta a la quimioterapia inicial con derivados del platino (NCT01847274). Se cerró la inscripción de pacientes en el ensayo y sus resultados están pendientes.
- En otros ensayos con inhibidores PARP, se ha estado explorando su función en la enfermedad resistente a los derivados del platino así como su función cuando se combinan con otros fármacos.
- El olaparib también se evaluó como fármaco único en un ensayo multicéntrico de fase ll para pacientes con mutaciones documentadas de la línea germinal de BRCA1 o BRCA2.[Nivel de evidencia C3] Este ensayo estuvo abierto para pacientes de cáncer de ovario resistente a los derivados del platino, cáncer de mama tratado antes con tres o más regímenes, cáncer de páncreas con administración previa de gemcitabina o cáncer de próstata tratado con terapia hormonal y una terapia sistémica. Olaparib se administró en dosis de 400 mg dos veces por día. El criterio principal de valoración fue la tasa de respuesta. Participaron 298 pacientes en total.
- La tasa total de respuesta fue del 26,2 %; la tasa de respuesta fue del 31,1 % en las pacientes de cáncer de ovario.[Nivel de evidencia C3]
La Administración de Alimentos y Medicamentos de los Estados Unidos (FDA) usó los datos de este ensayo para aprobar el olaparib para pacientes de cáncer de ovario con mutaciones conocidas de BRCA1 o BRCA2, y que fracasaron con los tres regímenes anteriores.
- En varios ensayos se combinó el olaparib con quimioterapia citotóxica u otras terapias biológicas.
- Se notó una prolongación de la SSP, pero no en la SG.
Recidiva con resistencia primaria o secundaria a los derivados del platino
Quimioterapia
Las recidivas clínicas que tienen lugar en un plazo de seis meses de haberse completado un régimen con un derivado del platino se consideran como recidivas con resistencia primaria o secundaria a los derivados del platino. Las antraciclinas (particularmente cuando se formulan como doxorrubicina liposomal pegilada), los taxanos, el topotecán y la gemcitabina se usan como fármacos únicos para estas recidivas con base en su actividad y sus índices terapéuticos favorables relacionados con los fármacos listados en el Cuadro 10. Esta larga lista enfatiza el beneficio marginal, si lo hubiera, que confieren estos fármacos. Se debe alentar a las pacientes con enfermedad que no responden a los derivados del platino a participar en ensayos clínicos.
Los fármacos utilizados para tratar la recidiva con resistencia primaria o secundaria a los derivados del platino son los siguientes:
- Paclitaxel.
Tradicionalmente, el tratamiento con paclitaxel proporcionó el primer fármaco que mostró una actividad constante en las pacientes con recidivas que presentan resistencia primaria o secundaria al platino. Las pacientes por lo general recibían paclitaxel en regímenes de inducción de primera línea. La repetición del tratamiento con paclitaxel, en especial mediante cronogramas semanales, presentaba una actividad comparable a la de otros fármacos. Si se presenta una neuropatía residual con la recidiva, esto puede ocasionar un cambio en la elección del tratamiento en favor de otros fármacos.
- Topotecán.
En estudios aleatorizados se indicó que el uso de topotecán permitía lograr resultados comparables a los obtenidos con paclitaxel.
Evidencia (topotecán):
- El topotecán se comparó con la doxorrubicina liposomal pegilada en un ensayo aleatorizado con 474 pacientes y se obtuvieron tasas de respuesta, SSP y SG similares en el momento del informe inicial. Las respuestas se produjeron, en su mayoría, en los subconjuntos resistentes al platino.
- En estudios de fase II, el topotecán intravenoso (IV) administrado los días 1 a 5 de un ciclo de 21 días rindió tasas de respuesta objetiva que oscilaron entre el 13 % y el 16,3 %, y otros resultados que fueron equivalentes o superiores al paclitaxel.
- Se notificaron respuestas objetivas en pacientes con enfermedad con resistencia primaria al platino.
- Después de la administración sigue una mielodepresión sustancial. Otros efectos tóxicos fueron náuseas, vómitos, alopecia y astenia. Se encuentran en evaluación algunos cronogramas y formulaciones orales.
- En un estudio de fase II, se asignó a 235 pacientes que no respondieron al tratamiento inicial con un régimen con derivados del platino, pero que no habían recibido antes paclitaxel o topotecán, a recibir topotecán en una infusión diaria de 30 minutos durante 5 días cada 21 días o paclitaxel como una infusión de 3 horas cada 21 días.[Nivel de evidencia B1]
- La tasa de respuesta objetiva general fue del 20,5 % en las pacientes asignadas al azar al tratamiento con topotecán y del 13,2 % en las pacientes asignadas al azar al tratamiento con paclitaxel (P = 0,138).
- Ambos grupos presentaron mielodepresión y efectos tóxicos gastrointestinales (GI). Se observaron con más frecuencia náuseas, vómitos, fatiga e infecciones después del tratamiento con topotecán, mientras que se observaron con mayor frecuencia alopecia, artralgia, mialgia y neuropatía después de la administración de paclitaxel.
- En un estudio de fase II se evaluó la combinación de topotecán semanal y bevacizumab dos veces por semana.
- Los resultados mostraron una tasa de respuesta objetiva del 25 % (todas respuestas parciales) en una población de pacientes resistentes al platino.
- Los efectos tóxicos más comunes de grado 3 y grado 4 fueron hipertensión, neutropenia y toxicidad GI, aunque no se presentaron perforaciones intestinales.
- Doxorrubicina liposomal pegilada.
Evidencia (doxorrubicina liposomal pegilada):
- En un estudio en fase II se administró doxorrubicina encapsulada IV 1 vez cada 21 a 28 días.
- Los resultados mostraron 1 respuesta completa y 8 respuestas parciales en 35 pacientes con enfermedad resistente a los derivados del platino o resistente al paclitaxel (tasa de respuesta, 25,7 %).
- En general, la doxorrubicina liposomal provoca pocos efectos secundarios agudos que no sean hipersensibilidad. Los efectos tóxicos más frecuentes (estomatitis y eritrodisestesia palmoplantar) se observaron en general después del primer ciclo y fueron más marcados después de la administración de dosis que excedían los 10 mg/m2 por semana. La neutropenia y las náuseas fueron mínimas y raras veces se presentó alopecia.
- En un ensayo aleatorizado con 474 pacientes de cáncer de ovario recidivante, se comparó la doxorrubicina liposomal con el topotecán.[Nivel de evidencia A1]
- Las tasas de respuesta (19,7 % vs. 17,0 %, P = 0,390), la SSP (16,1 vs. 17,0 semanas, P = 0,095) y la SG (60 vs. 56,7 semanas, P = 0,341) no difirieron de manera significativa entre los grupos de doxorrubicina liposomal y topotecán.[Nivel de evidencia A1]
- La supervivencia fue más prolongada en las pacientes con enfermedad sensible a los derivados del platino que recibieron doxorrubicina liposomal.
- En un estudio en fase II se administró doxorrubicina encapsulada IV 1 vez cada 21 a 28 días.
- Docetaxel.
Este medicamento ha mostrado actividad en pacientes tratadas previamente con paclitaxel y es una alternativa razonable al paclitaxel semanal en el entorno recidivante.
- Gemcitabina.
La gemcitabina, un antimetabolito concebido y aprobado para su uso en combinación con medicamentos de quimioterapia con derivados del platino, ha mostrado tener actividad como fármaco único. Se está explorando el uso de la gemcitabina combinada con medicamentos dirigidos al ciclo celular y otras combinaciones de medicamentos indicados para tipos de cáncer como el pancreático y pulmonar.
Evidencia (gemcitabina):
- Se notificaron varios ensayos de fase II con gemcitabina IV como fármaco único administrado los días 1, 8 y 15 de un ciclo de 28 días.
- La tasa de respuesta osciló del 13 % al 19 % en las pacientes evaluables.
- Se observaron respuestas en pacientes cuya enfermedad era resistente a los derivados del platino o al paclitaxel, así como en pacientes de enfermedad con gran masa tumoral.
- Los efectos tóxicos más comunes fueron leucopenia, anemia, y trombocitopenia. Muchas pacientes informaron que tuvieron síntomas pasajeros similares a los de la gripe y exantema después de la administración del medicamento. Otros efectos secundarios como las náuseas fueron en general muy leves.
- En un ensayo aleatorizado de gemcitabina versus doxorrubicina liposomal pegilada, no se observó inferioridad ni ventaja en el índice terapéutico de un fármaco sobre el otro.
- Se notificaron varios ensayos de fase II con gemcitabina IV como fármaco único administrado los días 1, 8 y 15 de un ciclo de 28 días.
- Pemetrexed.
El pemetrexed combinado con la gemcitabina ha tenido resultados poco convincentes en comparación con el uso de cada fármaco solo. Se aproximan más estudios dirigidos a los trastornos del ciclo celular frecuentes en ciertos subtipos genómicos del cáncer de ovario. En particular, se presume que la gemcitabina es más activa cuando hay pérdida del punto de control G1/S debido a mutaciones en TP53, amplificación de CCNE1, pérdida de RB1 o descenso del mRNA de CDKN2A.
Evidencia (pemetrexed):
- En un ensayo europeo aleatorizado con enmascaramiento doble de fase II con 102 pacientes, se evaluó el pemetrexed en dos dosis: estándar (500 mg/m2) versus una dosis alta (900 mg/m2) IV cada 3 semanas.
- La tasa de respuesta fue del 9,3 % para la dosis estándar y del 10,4 % para la dosis alta.
- El perfil de toxicidad favoreció la dosis estándar, con fatiga, náuseas y vómitos como los efectos secundarios más graves.
- En un estudio de fase II del GOG, se utilizó pemetrexed (900 mg/m2) IV cada 3 semanas en 51 pacientes con enfermedad recidivante resistente a los derivados del platino.
- La tasa de respuesta fue del 21 % en una población tratada con anterioridad de forma reiterada en la que el 39 % de las pacientes habían recibido cinco o más regímenes previos.
- La mielodepresión y el cansancio fueron los efectos secundarios graves más comunes.
- En un ensayo europeo aleatorizado con enmascaramiento doble de fase II con 102 pacientes, se evaluó el pemetrexed en dos dosis: estándar (500 mg/m2) versus una dosis alta (900 mg/m2) IV cada 3 semanas.
Quimioterapia o bevacizumab
-
Quimioterapia, con bevacizumab o sin este.
La Administración de Alimentos y Medicamentos de los Estados Unidos aprobó el uso de bevacizumab en combinación con doxorrubicina liposomal pegilada, paclitaxel o topotecán como resultado de los ensayos OCEANS y AURELIA. La información contenida en este enlace solo está disponible en inglés.
En el ensayo OCEANS [NCT00434642], se evaluó la función del bevacizumab en el tratamiento de las recidivas sensibles a los derivados del platino. Para obtener más información, consultar la sección Bevacizumab, otros fármacos dirigidos e inhibidores de la poli (ADP-ribosa)–polimerasa (PARP), con quimioterapia o sin esta.
Evidencia (bevacizumab con quimioterapia):
- El ensayo Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer (AURELIA NCT00976911) fue un ensayo aleatorizado sin enmascaramiento diseñado para evaluar el efecto de bevacizumab en la quimioterapia estándar en pacientes de cáncer de ovario recidivante resistente al platino. Las pacientes aptas tenían enfermedad resistente a los derivados del platino (progresión en el término de 6 meses de terminar con un régimen) y no se habían sometido antes a más de dos regímenes. Las pacientes con enfermedad con resistencia primaria a los derivados del platino (aquellas con progresión mientras recibían un régimen que contenía derivados del platino), así como aquellas con signos clínicos o radiológicos de compromiso intestinal no podían participar. Se indicó a las pacientes uno de los tres regímenes quimioterapéuticos siguientes según la preferencia del médico:
- Doxorrubicina liposomal pegilada 40 mg/m2 IV el día 1 cada 4 semanas.
- Paclitaxel 80 mg/m2 IV los días 1, 8, 15 y 22 cada 4 semanas.
- Topotecán 4 mg/m2 IV los días 1, 8 y 15 cada 4 semanas, o 1,25 mg/m2 IV 2, los días 1 a 5 cada 3 semanas.
Las pacientes se asignaron al azar para recibir quimioterapia sola o quimioterapia con bevacizumab (10 mg/kg cada 2 semanas o 15 mg/kg cada 3 semanas, si estaban en el cronograma de dosificación de 3 semanas). Se permitió pasar al régimen con bevacizumab en el momento de la progresión de la enfermedad a las pacientes del grupo de quimioterapia sola. El resultado principal fue la SSP, con tasa de respuesta, SG, inocuidad y calidad de vida como criterios secundarios de valoración. La inscripción incluyó a 361 pacientes con una mediana de seguimiento de 13,9 meses en el grupo de quimioterapia sola y de 13,0 meses en el grupo de quimioterapia y bevacizumab.
- Las pacientes en el grupo de bevacizumab exhibieron una SSP más prolongada (CRI, 0,48; IC 95 %, 0,38 a 0,60); la mediana de SSP fue de 3,4 meses en el grupo de quimioterapia sola versus 6,7 meses en el grupo de quimioterapia y bevacizumab.
- La tasa de respuesta objetiva fue del 12,6 % en el grupo de quimioterapia sola versus el 30,9 % en el grupo de quimioterapia y bevacizumab.
- No hubo una diferencia con significación estadística en la SG entre los regímenes (13,3 meses para la quimioterapia sola vs. 16,6 meses para la quimioterapia más bevacizumab).
- Las pacientes del grupo de quimioterapia y bevacizumab tuvieron un aumento de la incidencia de hipertensión y proteinuria, en comparación con las pacientes del grupo de quimioterapia sola.
- Solo se observó perforación GI en el 2 % de las pacientes que recibieron quimioterapia y bevacizumab; esto refleja los criterios de exclusión estrictos del estudio.
- El criterio principal de valoración para la porción de la calidad de vida del estudio fue del 15 % o más de mejoría absoluta en la porción síntoma abdominal y gastrointestinal de los módulos de evaluación en la semana 8 a la semana 9 del protocolo para las pacientes que recibieron quimioterapia y bevacizumab.[Nivel de evidencia A3] En el estudio se utilizaron los resultados notificados por las pacientes en el European Organisation for Research and Treatment of Cancer Ovarian Cancer Module 28 y el índice de síntomas del Functional Assessment of Cancer Therapy-Ovarian Cancer al inicio y cada 8 a 9 semanas hasta la progresión de la enfermedad.
Aunque hubo algunas limitaciones en el diseño del estudio, más pacientes en el grupo de quimioterapia y bevacizumab tuvieron un 15 % o más mejoría en sus puntajes GI en comparación con la situación inicial. En el grupo de quimioterapia más bevacizumab, 34 de 115 pacientes (29,6 %) exhibieron mejora versus 15 de 118 (12,7 %) pacientes que exhibieron mejora en el grupo de quimioterapia sola (diferencia, 16,9 %; IC 95 %, 6,1–27,6 %; P = 0,002).
Estos estudios confirman el efecto de mejorar la SSP cuando se añade bevacizumab a la quimioterapia para el cáncer de ovario. En el ensayo OCEANS, el CRI para la progresión fue incluso más prominente que en los ensayos de primera línea, y se observó un efecto significativo cuando se extendió la combinación bevacizumab-quimioterapia más allá de seis ciclos hasta la progresión.
En resumen, la mejora lograda con el bevacizumab en el riesgo relativo y las tasas de SSP en las recidivas sensibles a los derivados del platino y las recidivas resistentes a los derivados del platino fue de forma invariable mayor que la mejora alcanzada con la quimioterapia sola; sin embargo, se deben considerar los efectos tóxicos relacionados con el bevacizumab.
- El ensayo Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer (AURELIA NCT00976911) fue un ensayo aleatorizado sin enmascaramiento diseñado para evaluar el efecto de bevacizumab en la quimioterapia estándar en pacientes de cáncer de ovario recidivante resistente al platino. Las pacientes aptas tenían enfermedad resistente a los derivados del platino (progresión en el término de 6 meses de terminar con un régimen) y no se habían sometido antes a más de dos regímenes. Las pacientes con enfermedad con resistencia primaria a los derivados del platino (aquellas con progresión mientras recibían un régimen que contenía derivados del platino), así como aquellas con signos clínicos o radiológicos de compromiso intestinal no podían participar. Se indicó a las pacientes uno de los tres regímenes quimioterapéuticos siguientes según la preferencia del médico:
- Bevacizumab solo.
En tres estudios de fase ll se observó actividad para este anticuerpo del factor de crecimiento endotelial vascular.
- El primer estudio (GOG-0170D) incluyó a 62 pacientes que habían recibido solamente uno o dos tratamientos previos. Estas últimas pacientes habían recibido un régimen adicional con base en derivados del platino debido a un intervalo inicial de 12 meses o más luego de recibir regímenes de primera línea y tenían un estado funcional de 0 o 1. Las pacientes recibieron una dosis de 15 mg/kg cada 21 días.
- Se obtuvieron 2 respuestas completas y 11 respuestas parciales, una mediana de SSP de 4,7 meses y una SG de 17 meses. Esta actividad se notó tanto en los subconjuntos sensibles a los derivados del platino como en los que no respondían a los derivados del platino.
- En el segundo estudio solo se incluyó a pacientes con enfermedad resistente a los derivados del platino y se utilizó un cronograma de dosificación idéntico.
- El estudio se suspendió debido a que 5 de las 44 pacientes sufrieron perforaciones intestinales, una de las cuales fue mortal; se observaron siete respuestas parciales. Este aumento de riesgo de perforación intestinal se relacionó con tres o más tratamientos previos.[Nivel de evidencia C2]
- En el tercer estudio (CCC-PHII-45), se incluyó a 70 pacientes que recibieron 50 mg de ciclofosfamida oral diaria, además de bevacizumab (10 mg/kg cada 2 semanas).
- Se observaron 17 respuestas parciales y cuatro pacientes sufrieron perforaciones intestinales.
- El primer estudio (GOG-0170D) incluyó a 62 pacientes que habían recibido solamente uno o dos tratamientos previos. Estas últimas pacientes habían recibido un régimen adicional con base en derivados del platino debido a un intervalo inicial de 12 meses o más luego de recibir regímenes de primera línea y tenían un estado funcional de 0 o 1. Las pacientes recibieron una dosis de 15 mg/kg cada 21 días.
Inhibidores de puntos de control inmunitario
- Avelumab.
El avelumab, un anticuerpo dirigido al ligando de la proteína de muerte programada 1 (PD-L1), se estudió solo o en combinación con quimioterapia de doxorrubicina liposomal pegilada seguida por quimioterapia sola en pacientes con cáncer de ovario resistente a los derivados del platino o resistente al tratamiento.
Evidencia (avelumab):
- En el ensayo JAVELIN Ovarian 200 (NCT02580058), se inscribieron 556 pacientes entre enero de 2016 y marzo de 2016. Los pacientes se asignaron al azar a recibir avelumab con doxorrubicina liposomal pegilada, doxorrubicina liposomal pegilada sola o avelumab solo. Este estudio comenzó antes de que la FDA aprobara la combinación de bevacizumab y doxorrubicina liposomal pegilada en el ensayo AURELIA.
- Los resultados de la SSP y la SG no mostraron superioridad frente a la doxorrubicina liposomal pegilada. La mediana de la SSP fue de 3,7 meses (IC 95 %, 3,3–5,1) para los pacientes que recibieron el tratamiento combinado, de 3,5 meses (2,1–4,0) para los pacientes que recibieron doxorrubicina liposomal pegilada sola y de 1,9 meses (1,8–1,9) para los pacientes que recibieron avelumab solo. La mediana de la SG fue de 15,7 meses (IC 95 %, 12,7–18,7) para los pacientes que recibieron el tratamiento combinado, de 13,1 meses (11,8–15,5) para los pacientes que recibieron doxorrubicina liposomal pegilada sola y de 11,8 meses (8,9–14,1) para los pacientes que recibieron avelumab solo.
- En el ensayo JAVELIN Ovarian 200 (NCT02580058), se inscribieron 556 pacientes entre enero de 2016 y marzo de 2016. Los pacientes se asignaron al azar a recibir avelumab con doxorrubicina liposomal pegilada, doxorrubicina liposomal pegilada sola o avelumab solo. Este estudio comenzó antes de que la FDA aprobara la combinación de bevacizumab y doxorrubicina liposomal pegilada en el ensayo AURELIA.
- Durvalumab.
En estudios de fase temprana, se evaluó el uso de otros inhibidores de puntos de control inmunitario (por ejemplo, durvalumab) con doxorrubicina liposomal pegilada en pacientes con enfermedad recidivante, resistente a los derivados del platino.
Evidencia (durvalumab):
- En un ensayo de fase I/II (NCT02431559) publicado en forma de resumen, 40 pacientes a los que se les programó recibir doxorrubicina liposomal pegilada también recibieron durvalumab.
- A los 6 meses, la tasa de la SSP fue del 47,7 % (cuando se evaluó según el protocolo).
- La tasa de respuesta de la SG fue del 22,5 %, 4 pacientes alcanzaron una respuesta completa y 5 pacientes alcanzaron una respuesta parcial. La mediana de la SSP fue de 5,5 meses (0,3–28,8+) y la mediana de la SG fue de 17,6 meses (1,7–23.5+).
- En un ensayo de fase I/II (NCT02431559) publicado en forma de resumen, 40 pacientes a los que se les programó recibir doxorrubicina liposomal pegilada también recibieron durvalumab.
Otros fármacos utilizados para tratar recidivas con muestras de resistencia primaria o secundaria a los derivados del platino (la eficacia no está
No se confirmó que los fármacos incluidos en el Cuadro 10 tengan actividad en un entorno resistente a los derivados del platino, o tengan un índice terapéutico menos deseable y un nivel de evidencia inferior a C3.
| Fármacos | Clase de fármaco | Efectos tóxicos importantes | Comentarios |
|---|---|---|---|
| Etopósido | Inhibidor de la topoisomerasa II | Mielodepresión; alopecia | Administración oral; la leucemia poco frecuente disminuye la aceptabilidad y opaca el interés |
| Ciclofosfamida y varias bicloroetilaminas | Alquilantes | Mielodepresión; alopecia (solo con las oxazafosforinas) | Leucemia y cistitis; actividad incierta después de los derivados del platino |
| Hexametilmelamina (altretamina) | Desconocido, pero probablemente profármacos alquilantes | Emesis y efectos tóxicos neurológicos | Administración oral; actividad incierta después de los derivados del platino |
| Irinotecán | Inhibidor de la topoisomerasa I | Diarrea y otros síntomas gastrointestinales | Resistencia cruzada al topotecán |
| Oxaliplatino | Derivados del platino | Neuropatía, emesis, mielodepresión | Resistencia cruzada a los derivados del platino habituales, pero menor |
| Vinorelbina | Inhibidor mitótico | Mielodepresión | Actividad errática |
| Fluorouracilo y capecitabina | Antimetabolitos de fluoropirimidina | Síntomas gastrointestinales y mielodepresión | La capecitabina es oral; puede ser útil para tumores mucinosos |
| Tamoxifeno | Antiestrógeno | Tromboembolia | Administración oral; actividad mínima, quizás más en subconjuntos |
Ensayos clínicos en curso
Realizar una búsqueda avanzada en inglés de los ensayos clínicos sobre cáncer auspiciados por el NCI que ahora aceptan pacientes. La búsqueda se puede simplificar por ubicación del ensayo, tipo de tratamiento, nombre del fármaco y otros criterios. También se dispone de información general sobre los ensayos clínicos.
References
- Ozols RF, Bundy BN, Greer BE, et al.: Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 21 (17): 3194-200, 2003.
- Rustin GJ, van der Burg ME, Griffin CL, et al.: Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet 376 (9747): 1155-63, 2010.
- Stark DP, Cook A, Brown JM, et al.: Quality of life with cediranib in relapsed ovarian cancer: The ICON6 phase 3 randomized clinical trial. Cancer 123 (14): 2752-2761, 2017.
- Hoskins WJ, Rubin SC, Dulaney E, et al.: Influence of secondary cytoreduction at the time of second-look laparotomy on the survival of patients with epithelial ovarian carcinoma. Gynecol Oncol 34 (3): 365-71, 1989.
- Parmar MK, Ledermann JA, Colombo N, et al.: Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet 361 (9375): 2099-106, 2003.
- Shi T, Zhu J, Feng Y, et al.: Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 22 (4): 439-449, 2021.
- Coleman RL, Brady MF, Herzog TJ, et al.: Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 18 (6): 779-791, 2017.
- Du Bois A, Sehouli J, Vergote I, et al.: Randomized phase III study to evaluate the impact of secondary cytoreductive surgery in recurrent ovarian cancer: final analysis of AGO DESKTOP III/ENGOT-ov20. [Abstract] J Clin Oncol 2020; 38(15)(suppl): 6000. doi:10.1200/JCO.2020.38.15_suppl.6000. Available online. Last accessed October 28, 2022.
- van de Laar R, Zusterzeel PL, Van Gorp T, et al.: Cytoreductive surgery followed by chemotherapy versus chemotherapy alone for recurrent platinum-sensitive epithelial ovarian cancer (SOCceR trial): a multicenter randomised controlled study. BMC Cancer 14: 22, 2014.
- van de Laar R, Kruitwagen RF, Zusterzeel PL, et al.: Correspondence: Premature Stop of the SOCceR Trial, a Multicenter Randomized Controlled Trial on Secondary Cytoreductive Surgery: Netherlands Trial Register Number: NTR3337. Int J Gynecol Cancer 27 (1): 2, 2017.
- Monk BJ, Herzog TJ, Kaye SB, et al.: Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer. J Clin Oncol 28 (19): 3107-14, 2010.
- Pfisterer J, Plante M, Vergote I, et al.: Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 24 (29): 4699-707, 2006.
- Wagner U, Marth C, Largillier R, et al.: Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum-sensitive ovarian cancer patients. Br J Cancer 107 (4): 588-91, 2012.
- Bolis G, Scarfone G, Giardina G, et al.: Carboplatin alone vs carboplatin plus epidoxorubicin as second-line therapy for cisplatin- or carboplatin-sensitive ovarian cancer. Gynecol Oncol 81 (1): 3-9, 2001.
- Cantù MG, Buda A, Parma G, et al.: Randomized controlled trial of single-agent paclitaxel versus cyclophosphamide, doxorubicin, and cisplatin in patients with recurrent ovarian cancer who responded to first-line platinum-based regimens. J Clin Oncol 20 (5): 1232-7, 2002.
- Pfisterer J, Shannon CM, Baumann K, et al.: Bevacizumab and platinum-based combinations for recurrent ovarian cancer: a randomised, open-label, phase 3 trial. Lancet Oncol 21 (5): 699-709, 2020.
- Pignata S, Lorusso D, Joly F, et al.: Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: a randomised, phase 3 trial. Lancet Oncol 22 (2): 267-276, 2021.
- Muggia FM: Overview of carboplatin: replacing, complementing, and extending the therapeutic horizons of cisplatin. Semin Oncol 16 (2 Suppl 5): 7-13, 1989.
- Piccart MJ, Green JA, Lacave AJ, et al.: Oxaliplatin or paclitaxel in patients with platinum-pretreated advanced ovarian cancer: A randomized phase II study of the European Organization for Research and Treatment of Cancer Gynecology Group. J Clin Oncol 18 (6): 1193-202, 2000.
- Markman M, Markman J, Webster K, et al.: Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: implications for patient management and clinical trial design. J Clin Oncol 22 (15): 3120-5, 2004.
- Gordon AN, Tonda M, Sun S, et al.: Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol 95 (1): 1-8, 2004.
- Raja FA, Counsell N, Colombo N, et al.: Platinum versus platinum-combination chemotherapy in platinum-sensitive recurrent ovarian cancer: a meta-analysis using individual patient data. Ann Oncol 24 (12): 3028-34, 2013.
- Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, et al.: Pegylated liposomal Doxorubicin and Carboplatin compared with Paclitaxel and Carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol 28 (20): 3323-9, 2010.
- Aghajanian C, Blank SV, Goff BA, et al.: OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 30 (17): 2039-45, 2012.
- Bryant HE, Schultz N, Thomas HD, et al.: Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434 (7035): 913-7, 2005.
- Farmer H, McCabe N, Lord CJ, et al.: Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434 (7035): 917-21, 2005.
- Ledermann J, Harter P, Gourley C, et al.: Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366 (15): 1382-92, 2012.
- Ledermann J, Harter P, Gourley C, et al.: Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 15 (8): 852-61, 2014.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al.: Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 18 (9): 1274-1284, 2017.
- Friedlander M, Gebski V, Gibbs E, et al.: Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. Lancet Oncol 19 (8): 1126-1134, 2018.
- Penson RT, Valencia RV, Cibula D, et al.: Olaparib Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. J Clin Oncol 38 (11): 1164-1174, 2020.
- Kaye SB, Lubinski J, Matulonis U, et al.: Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol 30 (4): 372-9, 2012.
- Penson R, Valencia RV, Colombo N, et al.: Final overall survival results from SOLO3: Phase III trial assessing olaparib monotherapy versus non-platinum chemotherapy in heavily pretreated patients with germline BRCA1- and/or BRCA2-mutated platinum-sensitive relapsed ovarian cancer. [Abstract] Gynecol Oncol 166 (Suppl 1) A-026, S19-20, 2022.
- LYNPARZA (olaparib): Important Prescribing Information. AstraZeneca, 2022. Available online. Last accessed October 19, 2022.
- Swisher EM, Lin KK, Oza AM, et al.: Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 18 (1): 75-87, 2017.
- Coleman RL, Oza AM, Lorusso D, et al.: Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390 (10106): 1949-1961, 2017.
- Ledermann JA, Oza AM, Lorusso D, et al.: Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial. Lancet Oncol 21 (5): 710-722, 2020.
- Oza AM, Lorusso D, Aghajanian C, et al.: Patient-Centered Outcomes in ARIEL3, a Phase III, Randomized, Placebo-Controlled Trial of Rucaparib Maintenance Treatment in Patients With Recurrent Ovarian Carcinoma. J Clin Oncol 38 (30): 3494-3505, 2020.
- Oza AM, Lisyanskaya AS, Fedenko AA, et al.: Overall survival results from ARIEL4: A phase III study assessing rucaparib vs chemotherapy in patients with advanced, relapsed ovarian carcinoma and a deleterious BRCA1/2 mutation. [Abstract] Ann Oncol 33 (Suppl 7) A-5180, S780, 2022.
- Moore KN, Secord AA, Geller MA, et al.: Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 20 (5): 636-648, 2019.
- ZEJULA (niraparib): Important Prescribing Information. GSK, 2022. Available online. Last accessed October 27, 2022.
- Mirza MR, Monk BJ, Herrstedt J, et al.: Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 375 (22): 2154-2164, 2016.
- Del Campo JM, Matulonis UA, Malander S, et al.: Niraparib Maintenance Therapy in Patients With Recurrent Ovarian Cancer After a Partial Response to the Last Platinum-Based Chemotherapy in the ENGOT-OV16/NOVA Trial. J Clin Oncol 37 (32): 2968-2973, 2019.
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al.: Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33 (3): 244-50, 2015.
- Liu JF, Barry WT, Birrer M, et al.: Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol 15 (11): 1207-14, 2014.
- Oza AM, Cibula D, Benzaquen AO, et al.: Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 16 (1): 87-97, 2015.
- Kohn EC, Sarosy G, Bicher A, et al.: Dose-intense taxol: high response rate in patients with platinum-resistant recurrent ovarian cancer. J Natl Cancer Inst 86 (1): 18-24, 1994.
- McGuire WP, Rowinsky EK, Rosenshein NB, et al.: Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med 111 (4): 273-9, 1989.
- Einzig AI, Wiernik PH, Sasloff J, et al.: Phase II study and long-term follow-up of patients treated with taxol for advanced ovarian adenocarcinoma. J Clin Oncol 10 (11): 1748-53, 1992.
- Thigpen JT, Blessing JA, Ball H, et al.: Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a Gynecologic Oncology Group study. J Clin Oncol 12 (9): 1748-53, 1994.
- Trimble EL, Adams JD, Vena D, et al.: Paclitaxel for platinum-refractory ovarian cancer: results from the first 1,000 patients registered to National Cancer Institute Treatment Referral Center 9103. J Clin Oncol 11 (12): 2405-10, 1993.
- ten Bokkel Huinink W, Gore M, Carmichael J, et al.: Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol 15 (6): 2183-93, 1997.
- Gordon AN, Fleagle JT, Guthrie D, et al.: Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 19 (14): 3312-22, 2001.
- Kudelka AP, Tresukosol D, Edwards CL, et al.: Phase II study of intravenous topotecan as a 5-day infusion for refractory epithelial ovarian carcinoma. J Clin Oncol 14 (5): 1552-7, 1996.
- Creemers GJ, Bolis G, Gore M, et al.: Topotecan, an active drug in the second-line treatment of epithelial ovarian cancer: results of a large European phase II study. J Clin Oncol 14 (12): 3056-61, 1996.
- Bookman MA, Malmström H, Bolis G, et al.: Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase II study in patients treated after prior chemotherapy that contained cisplatin or carboplatin and paclitaxel. J Clin Oncol 16 (10): 3345-52, 1998.
- McGonigle KF, Muntz HG, Vuky J, et al.: Combined weekly topotecan and biweekly bevacizumab in women with platinum-resistant ovarian, peritoneal, or fallopian tube cancer: results of a phase 2 study. Cancer 117 (16): 3731-40, 2011.
- Muggia FM, Hainsworth JD, Jeffers S, et al.: Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol 15 (3): 987-93, 1997.
- Berkenblit A, Seiden MV, Matulonis UA, et al.: A phase II trial of weekly docetaxel in patients with platinum-resistant epithelial ovarian, primary peritoneal serous cancer, or fallopian tube cancer. Gynecol Oncol 95 (3): 624-31, 2004.
- Friedlander M, Millward MJ, Bell D, et al.: A phase II study of gemcitabine in platinum pre-treated patients with advanced epithelial ovarian cancer. Ann Oncol 9 (12): 1343-5, 1998.
- Lund B, Hansen OP, Theilade K, et al.: Phase II study of gemcitabine (2',2'-difluorodeoxycytidine) in previously treated ovarian cancer patients. J Natl Cancer Inst 86 (20): 1530-3, 1994.
- Shapiro JD, Millward MJ, Rischin D, et al.: Activity of gemcitabine in patients with advanced ovarian cancer: responses seen following platinum and paclitaxel. Gynecol Oncol 63 (1): 89-93, 1996.
- Mutch DG, Orlando M, Goss T, et al.: Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol 25 (19): 2811-8, 2007.
- Yuan Y, Cohen DJ, Love E, et al.: Phase I dose-escalating study of biweekly fixed-dose rate gemcitabine plus pemetrexed in patients with advanced solid tumors. Cancer Chemother Pharmacol 68 (2): 371-8, 2011.
- Hensley ML, Larkin J, Fury M, et al.: A phase I trial of pemetrexed plus gemcitabine given biweekly with B-vitamin support in solid tumor malignancies or advanced epithelial ovarian cancer. Clin Cancer Res 14 (19): 6310-6, 2008.
- Vergote I, Calvert H, Kania M, et al.: A randomised, double-blind, phase II study of two doses of pemetrexed in the treatment of platinum-resistant, epithelial ovarian or primary peritoneal cancer. Eur J Cancer 45 (8): 1415-23, 2009.
- Miller DS, Blessing JA, Krasner CN, et al.: Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecologic Oncology Group. J Clin Oncol 27 (16): 2686-91, 2009.
- Pujade-Lauraine E, Hilpert F, Weber B, et al.: Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 32 (13): 1302-8, 2014.
- Stockler MR, Hilpert F, Friedlander M, et al.: Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol 32 (13): 1309-16, 2014.
- Liu JF, Cannistra SA: Emerging role for bevacizumab in combination with chemotherapy for patients with platinum-resistant ovarian cancer. J Clin Oncol 32 (13): 1287-9, 2014.
- Burger RA, Sill MW, Monk BJ, et al.: Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25 (33): 5165-71, 2007.
- Cannistra SA, Matulonis UA, Penson RT, et al.: Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 25 (33): 5180-6, 2007.
- Vasey PA, McMahon L, Paul J, et al.: A phase II trial of capecitabine (Xeloda) in recurrent ovarian cancer. Br J Cancer 89 (10): 1843-8, 2003.
- Monk BJ, Han E, Josephs-Cowan CA, et al.: Salvage bevacizumab (rhuMAB VEGF)-based therapy after multiple prior cytotoxic regimens in advanced refractory epithelial ovarian cancer. Gynecol Oncol 102 (2): 140-4, 2006.
- Kaye SB: Bevacizumab for the treatment of epithelial ovarian cancer: will this be its finest hour? J Clin Oncol 25 (33): 5150-2, 2007.
- Garcia AA, Hirte H, Fleming G, et al.: Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol 26 (1): 76-82, 2008.
- Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al.: Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol 22 (7): 1034-1046, 2021.
- O'Cearbhaill RE, Wolfer A, Disilvestro P: A phase I/II study of chemo-immunotherapy with durvalumab (durva) and pegylated liposomal doxorubicin (PLD) in platinum-resistant recurrent ovarian cancer (PROC). [Abstract] Ann Oncol 29 (suppl 8): A-945P, viii337, 2018. Also available online. Last accessed February 8, 2023.
Actualizaciones más recientes a este resumen (02/16/2023)
Los resúmenes del PDQ con información sobre el cáncer se revisan con regularidad y se actualizan a medida que se obtiene nueva información. Esta sección describe los cambios más recientes introducidos en este resumen a partir de la fecha arriba indicada.
Información general sobre el cáncer epitelial de ovario, el cáncer de trompas de Falopio y el cáncer primario de peritoneo
Se actualizaron las estadísticas con el número estimado de casos nuevos y defunciones para 2023 (se citó a la American Cancer Society como referencia 5).
El Consejo editorial del PDQ sobre el tratamiento para adultos es responsable de la redacción y actualización de este resumen y mantiene independencia editorial respecto del NCI. El resumen refleja una revisión independiente de la bibliografía médica y no representa las políticas del NCI ni de los NIH. Para obtener más información sobre las políticas relativas a los resúmenes y la función de los consejos editoriales del PDQ responsables de su actualización, consultar Información sobre este resumen del PDQ e Información del PDQ® sobre el cáncer dirigida a profesionales de la salud.
Información sobre este resumen del PDQ
Propósito de este resumen
Este resumen de información del PDQ sobre el cáncer dirigido a profesionales de la salud proporciona información integral revisada por expertos y basada en la evidencia sobre el tratamiento del cáncer epitelial de ovario, de trompas de Falopio y primario de peritoneo. El objetivo es servir como fuente de información y ayuda para los profesionales clínicos durante la atención de pacientes. No ofrece pautas ni recomendaciones formales para tomar decisiones relacionadas con la atención sanitaria.
Revisores y actualizaciones
El Consejo editorial del PDQ sobre el tratamiento para adultos, que mantiene independencia editorial respecto del Instituto Nacional del Cáncer (NCI), revisa este resumen de manera periódica y, en caso necesario, lo actualiza. Este resumen es el resultado de una revisión bibliográfica independiente y no constituye una declaración de política del NCI ni de los Institutos Nacionales de la Salud (NIH).
Cada mes, los integrantes de este consejo revisan los artículos publicados recientemente para determinar lo siguiente:
- Si el artículo se debe analizar en una reunión del consejo.
- Si conviene añadir texto acerca del artículo.
- Si se debe reemplazar o actualizar un artículo que ya se citó.
Los cambios en los resúmenes se deciden mediante consenso de los integrantes del consejo después de evaluar la solidez de la evidencia de los artículos publicados y determinar la forma de incorporar el artículo en el resumen.
El revisor principal del sumario sobre Tratamiento del cáncer epitelial de ovario, de trompas de Falopio y primario de peritoneo es:
- Olga T. Filippova, MD (Memorial Sloan-Kettering Cancer Center )
Cualquier comentario o pregunta sobre el contenido de este resumen se debe enviar al Servicio de Información de Cáncer del Instituto Nacional del Cáncer. Por favor, no enviar preguntas o comentarios directamente a los integrantes del consejo, ya que no responderán consultas de manera individual.
Niveles de evidencia
Algunas de las referencias bibliográficas de este resumen se acompañan del nivel de evidencia. El propósito de esto es ayudar al lector a evaluar la solidez de la evidencia que respalda el uso de ciertas intervenciones o abordajes. El Consejo editorial del PDQ sobre el tratamiento para adultos emplea un sistema de jerarquización formal para asignar los niveles de evidencia científica.
Permisos para el uso de este resumen
PDQ (Physician Data Query) es una marca registrada. Se autoriza el uso del texto de los documentos del PDQ; sin embargo, no se podrá identificar como un resumen de información sobre cáncer del PDQ del NCI, salvo que el resumen se reproduzca en su totalidad y se actualice de manera periódica. Por otra parte, se permitirá que un autor escriba una oración como “En el resumen del PDQ del NCI de información sobre la prevención del cáncer de mama se describen, de manera concisa, los siguientes riesgos: [incluir fragmento del resumen]”.
Se sugiere citar la referencia bibliográfica de este resumen del PDQ de la siguiente forma:
PDQ® sobre el tratamiento para adultos. PDQ Tratamiento del cáncer epitelial de ovario, de trompas de Falopio y primario de peritoneo. Bethesda, MD: National Cancer Institute. Actualización:
Las imágenes en este resumen se reproducen con autorización del autor, el artista o la editorial para uso exclusivo en los resúmenes del PDQ. La utilización de las imágenes fuera del PDQ requiere la autorización del propietario, que el Instituto Nacional del Cáncer no puede otorgar. Para obtener más información sobre el uso de las ilustraciones de este resumen o de otras imágenes relacionadas con el cáncer, consultar Visuals Online, una colección de más de 2000 imágenes científicas.
Cláusula sobre el descargo de responsabilidad
Según la solidez de la evidencia, las opciones de tratamiento se clasifican como “estándar” o “en evaluación clínica”. Estas clasificaciones no se deben utilizar para justificar decisiones sobre reembolsos de seguros. Para obtener más información sobre la cobertura de seguros, consultar la página Manejo de la atención del cáncer en Cancer.gov/espanol.
Comuníquese con el Instituto Nacional del Cáncer
Para obtener más información sobre las opciones para comunicarse con el NCI, incluso la dirección de correo electrónico, el número telefónico o el chat, consultar la página del Servicio de Información de Cáncer del Instituto Nacional del Cáncer.